Methanol and ethanol form an ideal solution. Compute vapor-liquid equilibrium data and prepare plots of x-y and T-x-y at 1 atm pressure. The following pure component vapor pressure data is given:
|
Vapor pressure, mm Hg |
200 |
400 |
760 |
1520 |
|
Temperature oC for ethanol |
48.4 |
62.5 |
78.4 |
97.5 |
|
Temperature oC for methanol |
34.8 |
49.9 |
64.7 |
84 |
What value of relative volatility will you recommend for this system?
Calculations:
Boiling point for the solution will range from the pure component boiling point of low boiling component to the pure component boiling point of high boiling component.
In this case, the boiling point of solution will range from 64.7oC to 78.4oC.
According to Clasius-Clapeyron equation, for short ranges of temperatures ln P Vs 1/T (temperature in Kelvin) is a straight line.
We can utilize this linear relationship fact, for interpolation of vapor pressure for various temperatures.
For methanol and ethanol we want to have the vapor pressure data for the temperature range of 64.7 to 48.4oC.
Interpolating equation for methanol: (using the data for T of 64.7 and 84oC)
(ln 760 - ln PA) / (ln 760 - ln 1520) = (1/337.7 - 1/T) / (1/337.7 - 1/357)
Interpolating equation for ethanol: (using the data for T of 62.5 and 78.4oC)
(ln 400 - ln PB) / (ln 400 - ln 760) = (1/335.5 - 1/T) / (1/335.5 - 1/351.4)
The vapor pressure data for various pressures are calculated using the above equations and tabulated as follows:
| T, oC | T, Kelvin | ln PA | PA | ln PB | PB |
| 64.7 | 337.7 | 6.6333 | 760 | 6.0839 | 439 |
| 68 | 341 | 6.7574 | 860 | 6.2203 | 503 |
| 71 | 344 | 6.8681 | 961 | 6.3420 | 568 |
| 74 | 347 | 6.9769 | 1072 | 6.4616 | 640 |
| 75.5 | 348.5 | 7.0307 | 1131 | 6.5206 | 679 |
| 78.4 | 351.4 | 7.1332 | 1253 | 6.6333 | 760 |
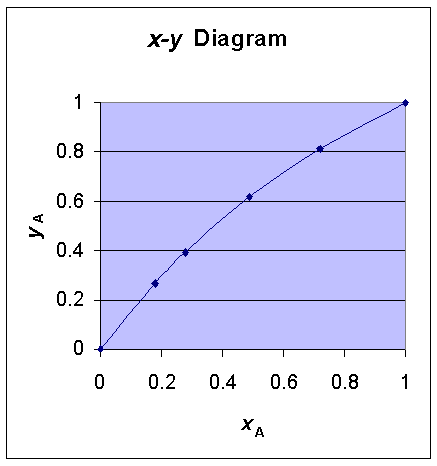
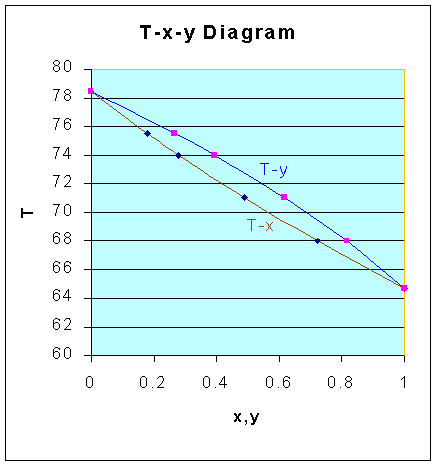
Using these vapor pressure - temperature data, and from the relation:
x
A = (Pt - PB)/(PA - PB) and yA = PAxA/Ptand Relative volatility, a = PA/PB
x-y
plot T-x-y plot data are calculated as follows:|
T, oC |
PA, mm Hg |
PB, mm Hg |
x A |
y A |
a |
| 64.7 | 760 | 439 | 1 | 1 | 1.73 |
| 68 | 860 | 503 | 0.720 | 0.815 | 1.71 |
| 71 | 961 | 568 | 0.489 | 0.618 | 1.69 |
| 74 | 1072 | 640 | 0.278 | 0.392 | 1.68 |
| 75.5 | 1131 | 679 | 0.179 | 0.267 | 1.67 |
| 78.4 | 1253 | 760 | 0 | 0 | 1.65 |
|
Average a = 1.69 |
|||||

The recommended relative volatility for the system is 1.69.
Last Modified on: 01-May-2024
Chemical Engineering Learning Resources - msubbu
e-mail: learn[AT]msubbu.academy
www.msubbu.in