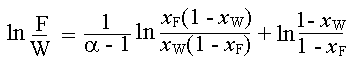
100 moles of Benzene (A) and Toluene mixture containing 50% (mole) of Benzene is subjected to a differential distillation at atmospheric pressure till the composition of benzene in the residue is 33%. Calculate the total moles of the mixture distilled. Average relative volatility may be assumed as 2.16
Calculations:
For system of constant relative volatility, the following equation is obtained after the simplification of Rayleigh's equation for differential distillation:
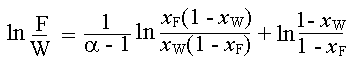
In the above equation, F is feed; W is residue; xF is composition of feed; xW is composition of residue; and a is relative volatility.
Given: xF = 0.5; xW = 0.33; F = 100; a = 2.16
Substituting these in the above equation,
ln (100/W) = (1/1.16) x ln {[(0.5(1 - 0.33)]/[(0.33(1 - 0.5)]} + ln [(1 - 0.33)/(1 - 0.5)]
ln (100/W) = 0.862 x ln (2.03) + ln (1.34)
ln (100/W) = 0.862 x 0.708 + 0.2927
ln (100/W) = 0.903
100/W = e0.903
100/W = 2.467
W = 100/2.467 = 40.54 mole
Moles of mixture distilled
= F - W = 100 - 40.54 = 59.46The above can also be obtained by using the general form of Rayleigh's equation and by graphical method. This is given below.
The equilibrium curve relationship is given by
y* = ax/(1 + x(a - 1)) = 2.16x/(1 + 1.16x) à 1
For differential distillation, the following Rayleigh's equation is applicable:
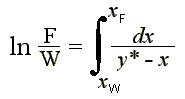 à 2
à 2
By using Equn.1 the following equilibrium data is obtained: (for the region of x = 0.33 to 0.5)
|
x |
y* |
|
0.33 |
0.515 |
|
0.36 |
0.549 |
|
0.39 |
0.580 |
|
0.42 |
0.610 |
|
0.45 |
0.639 |
|
0.48 |
0.666 |
|
0.5 |
0.684 |
For the above data the following table of x Vs.1/(y* - x) is obtained:
|
x |
1/(y* - x) |
|
0.33 |
5.392 |
|
0.36 |
5.304 |
|
0.39 |
5.263 |
|
0.42 |
5.263 |
|
0.45 |
5.301 |
|
0.48 |
5.377 |
|
0.5 |
5.448 |
Using the above data a graph of x Vs.1/(y* - x) is drawn between the limits of x = 0.33 to 0.5.
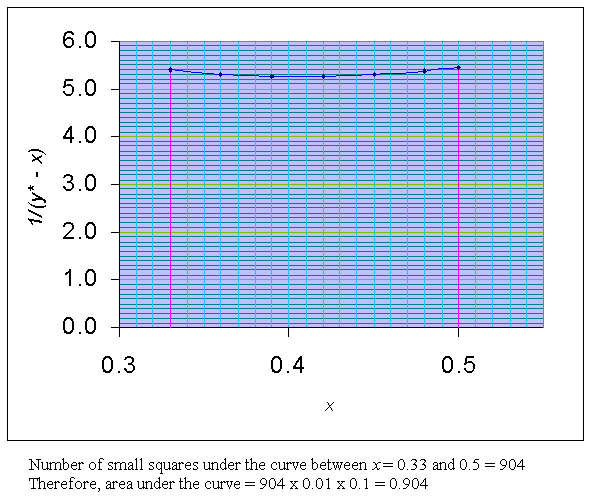
Area under the curve between the limits of x = 0.33 to 0.5, is = 0.904
i.e.,
ln (F/W) = 0.904
e0.904 = F/W
2.4695 = F/W
Given F = 100 moles. Therefore,
W = 100 / 2.4695 = 40.5 mole.
Therefore, moles of mixture distilled = F - W = 100 - 40.5 = 59.5
Last Modified on: 01-May-2024
Chemical Engineering Learning Resources - msubbu
e-mail: learn[AT]msubbu.academy
www.msubbu.in