(b) Explain
(i) System and Surroundings (3)
(ii) Macroscopic and Microscopic aspects (3)
Or
(c) Explain the work required in the case of Isothermal and Adiabatic process (8)
(d) Calculate the work done when 65.38 gram of zinc dissolves in HCl (i) in a open beaker (ii) a closed beaker at 300 K (4)
(b) Derive van der Waal's equation of stae (7)
Or
(c) Show that in multistage compression for minimum work, the inter-stage pressure is the geometric mean of the initial and final pressures (8)
(d) Methane is to be compressed in an adiabatic compressor with inter-stage cooling from an initial pressure of 1.03 x 105 N/m2 to 201.06 x 105 N/m2. If the number of stages in the compressor is 4, what would be the compression ratio for optimal operation? (4)
(b) Show that 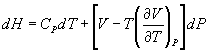
Or
(c) What is chemical potential? (3)
(d) Explain (i) Helmholtz free energy (ii) Gibbs free energy (iii) Joule Thomson Coefficient (3 x 3 = 9)
(b) For a binary mixture of 'A' and 'B' activity coefficient data for 'A' are available over the entire composition range, while one data is available for 'B'. Show how you would determine activity coefficient for 'B' over the entire range of composition (4)
Or
(c) Explain equilibrium curves and B.P. diagrams (6)
(d) An experimental determination of a VLE state for the ethanol, toluene system gave the following results:
Standard V.P. at 45oC, Ethanol = 173 mm Hg, Toluene = 75.4 mm Hg, x1 = 0.3; y1 = 0.634; PT = 183 mm Hg. Calculate (i) the liquid phase activity coefficient (ii) does the liquid phase exhibit positive or negative deviation from the ideal solution behavior? (6)
(b) Compute the equilibrium constant for the water gas reaction at 540oC (6)
CO + H2O à CO2 + H2
Data:
|
|
D Hf cal/gmol |
|
CO |
-26,420 |
|
H2O |
-57,800 |
|
CO2 |
-94,050 |
|
H2 |
0 |
Or
(c) For the reaction SO2 + 1/2 O2 à SO3 in equilibrium at 900 K, what pressure is required for a 90% conversion of SO2 if the initial mixture is equimolar in the reactants. Take Ka = 1.3 at 900 K.