- Determine the activation energy and the frequency factor from the following data for the bimolecular formation of methyl ethyl ether.
ToC 0 6 12 18 24 30
10 k, 5.6 11.8 24.5 48.8 100 208
litres/gmol.sec
what do you understand by these two terms?
- The homogeneous gas-phase reaction A à
3B has a rate constant of k = 0.5 min-1. Obtain an expression for the total pressure in a constant volume reactor as a function of time t (min) and the initial pressure po. What will be the effect of 50% inerts present initially along with pure A?
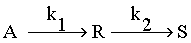 Under appropriate conditions, the following reaction takes place.
Under appropriate conditions, the following reaction takes place. With k1 = k2 = 0.1 min-1. R is to be produced from 1000 litres/hour of feed with CAO = 1 gmol/litre and CRO = CSO = 0. What size of plug flow reactor will maximize the yield of R? What is the concentration of R in the effluent stream of this of this reactor?
- A reactor decomposing by a second order mechanism is passed through a PFR and CSTR of equal volumes in series. The effluent from the CSTR contains 1% of the original reactant. How much of the original reactant would be left in the process stream, if it passed through the CSTR and then through the PFR? There is no change in density or temperature of the process stream.
- Establish the kinetics of the thermal decomposition of Nitrous oxide from the following data and find the reaction rate at 990oC and an initial pressure of 200 mm Hg.
t, min 20 53 100
% decomposition 23 50 73
- An elementary liquid phase reaction (irreversible first order) A à
R, takes place in a PFR and the conversion is 96%. If a mixed flow reactor of 10 times as large as the PFR is hooked up in parallel with the existing unit, by what fraction could the production be increased for the same 96% conversion?
- Determine the activation energy and frequency factor using Arrhenius and Transition state theories from the following data:
k, min-1 0.001 0.05
T oC 0.0 100.0
- Sulfuryl chloride vapors are heated in a closed vessel for 45 min at 320oC and 1 atm initial pressure. The dissociation of SO2Cl2 to SO2 and Cl2 is a first order reaction. The reaction velocity constant is 0.00130 min-1. Calculate the percentage decomposition of SO2Cl2 and the time required to decompose 80% of SO2Cl2.
- A homogeneous liquid phase reaction A à
R takes place with 50% conversion in a mixed reactor. The reaction is elementary second order.
- What will be the conversion if the original reactor is replaced by one which is six times as large as the original reactor - all else remaining unchanged?
- What will be the conversion if the original reactor is replaced by a plug flow reactor of equal size - all else remaining unchanged?
- A liquid is to be hydrolyzed in three mixed reactors connected in series. If each reactor has a volume of 2 litres and the feed rate to the first reactor is 500 cc/min, determine the percent hydrolysis accomplished in the three reactors.
Data: Rate of hydrolysis = r = 0.16 gmol/cc.min
- The thermal decomposition of nitrous oxide (N2O) in the gas phase at 1030oK is studied in a constant volume vessel at various initial pressures of N2O. The half-life data so obtained are as follows:
po (mm Hg) 52.5 139 290 360
t1/2 (sec) 860 470 255 212
2N2O à
2N2 + O2
Determine the rate equation that fits the data.
- The decomposition of NO2 follows a second order rate equation. Data at different temperatures are as follows:
T (K) 592 603 627 651.5 656
k (cm3/gmol.sec)522 755 1700 4020 5030
Compute the energy of activation E from the data. The reaction is 2NO2 à
2NO + O2
- Two stirred tanks of volume V1 and 2V1 are available for carrying out a first order irreversible reaction at constant density and temperature. If the flow rate of the feed stream is Q, which of the following arrangements would give the highest production rate of the product?
- Parallel operation of the two reactors, with equal average residence times.
- Parallel operation, with different average residence times.
- Series operation, with feed stream entering the larger reactor.
- Series operation, with feed entering the smaller reactor.
- The gas phase decomposition of A takes place according to the irreversible reaction, A à
3P. The kinetics of the reaction was studied by measuring the increase in pressure in a constant volume reaction vessel. At 504oC and an initial pressure of 312 mm Hg, the following data were obtained:
Time (Sec) 390 777 1195 3155 a
Total pressure (mm Hg) 408 488 562 779 931
- Test for a first order reaction.
- Calculate the value of the specific reaction rate at 504oC.
- The reaction A d
B ‡
C has the rate equations.
rA = 0.4 CA, rB = 0.3 CB - 0.4 CC
Three tanks of different sizes are available. At the desired production rate, the residence times of 5 min, 10 min and 15 min, are maintained in the successive stages of the CSTR battery. The feed concentrations are CAo = 0.9 ; CBo = 1.2 and CCo = 0
Find the concentration of B in the effluent from each tank.
- Find the specific reaction rate and order of reaction for thermal decomposition of nitrous oxide on gold at 990oC and initial pressure of 200 mm Hg.
t, min 20 53 100
% decomposition 32& 50 73
- The decomposition of phosphine is irreversible and first order at 650oC.
4PH3(g) à
P4(g) + 6H2(g)
The rate constant (sec-1) is reported as
log k = (-18993/T ) + 2 log T + 12.13
where T is in oK.
In a closed vessel (constant volume) initially containing phosphine at 1 atm pressure, what will be the pressure after 50, 100 and 500 sec. The temperature is maintained at 650oC.
- The kinetics of an aqueous phase decomposition of A is investigated in two CSTR's in series, the first having half the volume of the second. At steady state with a feed concentration of 4 gmol/lit., and mean residence time of 65 sec in the second reactor, the concentration of feed to second from the first is found to be 2 gmol/lit., while that of the stream leaving the second is 1 gmol/lit. Find the suitable kinetic rate expression.
- Two small samples of solids are introduced into a
constant environment oven and kept there for 1 hour. Under these
conditions 4 mm particles are 58% converted and 2 mm particles are 87.5%
converted. Find
- the rate controlling mechanism for the conversion of solids.
- Time needed for the complete conversion of 1 mm particles in this
oven.
- The catalytic reaction A Û
M + N takes place in a reactor where the effect of diffusive mass
transfer may be neglected. The following steps may be considered
- Adsorption of A
- Adsorbed A reacts with a vacant active centre to produce adsorbed M
and N
- Desorption of M and N.
Assuming the surface reaction is rate controlling, develop the overall
rate equation.
- The solid-catalyzed decomposition of gaseous A proceeds as
follows:
A à
R -rA = kCA2
A tubular pilot plant reactor packed with 2 litres of catalyst is
fed 2 m3/hr of pure A at 300oC and 20 atm.
Conversion of reactant is 65%.
In a large plant, it is desired to treat 100 m3/hr of feed
gases at 40 atm and 300oC containing 60% A and 40% inerts to
obtain 85% conversion of A. Find the internal volume of the reactor
required.
- A batch of solids of uniform size is treated by gas in a uniform
environment. Solid is converted to give a non-flaking product according to
the shrinking core model. Conversion is about 7/8 for a reaction time of
one hour, and conversion is complete in two hours. What mechanism is rate
controlling?
- The following kinetic data on the reaction A à
R are obtained in an experimental packed bed reactor using various
amounts of catalyst and a fixed feed rate of FAO = 10 kmol/hr.
W kg. catalyst 1 2 3 4 5
6 7
XA 0.12 0.2 0.27 0.33 0.37 0.41
0.44
Find the reaction rate at 40% conversion.