GATE-CH-2007-32-td-2mark
Which one of the following represents the Carnot cycle (ideal engine) ?
Entropy change for an irreversible process, taking into account both the system and surroundings together, is:
Mixing of two fluids is a
The shape of \(T\)-\(S\) diagram for Carnot cycle is
A reversible heat transfer demands:
When a system executes an irreversible cycle:
The second law of thermodynamics states that
The kinetic energy of gas molecules is zero at
A closed system is cooled reversibly from 100\(^\circ \)C to 50\(^\circ \)C. If no work is done on the system
A system undergoes a change from a given initial state to a given final state either by an irreversible process or by a reversible process. Then ---------------
where \(\Delta S_I\) and \(\Delta S_R\) are the entropy changes of the system for the irreversible and reversible processes, respectively.
On a \(P\)-\(V\) diagram of an ideal gas, suppose a reversible adiabatic line intersects a reversible isothermal line at point \(A\). Then at point \(A\), the slope of the reversible adiabatic line \((\partial P/\partial V)_S\) and the slope of the reversible isothermal line \((\partial P/\partial V)_T\) are related as _________ (where \(\gamma = C_P/C_V\))
The following heat engine produces power of 100,000 kW. The heat engine operates between 800 K and 300 K. It has a thermal efficiency equal to 50% of that of the Carnot engine for the same temperatures. The rate at which heat is absorbed from the hot reservoir is
A cyclic engine exchanges heat with two reservoirs maintained at 100 and 300oC , respectively. The maximum work (in J) that can be obtained from 1000 J of heat extracted from the hot reservoir is
Which one of the following statements is TRUE?
A heat engine operates at 75% of the maximum possible efficiency. The ratio of the heat source temperature (in K) to the heat sink temperature (in K) is 5/3. The fraction of the heat supplied that is converted to work is
For the isentropic expansion of an ideal gas from the initial conditions \(P_1,T_1\) to the final conditions \(P_2,T_2\), which ONE of the following relations is valid (\(\gamma = C_P/C_V\))
Which one of the following represents the Carnot cycle (ideal engine) ?
The change in entropy of the system, \(\Delta S_{\text {sys}}\), undergoing a cyclic irreversible process is
In a parallel flow heat exchanger operating under steady state, hot liquid enters at a temperature \(T_{\text {h,in}}\) and leaves at \(T_{\text {h,out}}\). Cold liquid enters at a temperature of \(T_{\text {c,in}}\) and leaves at a temperature \(T_{\text {c,out}}\). Neglect any heat loss from the heat exchanger to the surrounding. If \(T_{\text {h,in}} \gg T_{\text {c,in}}\), then for a given time interval, which ONE of the following statements is true?
In a process occurring in a closed system \(F\), the heat transferred from \(F\) to the surroundings \(E\) is 600 J. If the temperature of \(E\) is 300 K and that of \(F\) is in the range 380-400 K, the entropy changes of the surroundings \((\Delta S_E)\) and system \((\Delta S_F)\), in J/K, are given by
Water is heated at atmospheric pressure from 40oC to 80oC using two different processes. In process I, the heating is done by a source at 80oC. In process II, the water is heated from 40oC to 60oC by a source at 60oC, and then from 60oC to 80oC by another source at 80oC .
Identify the correct statement.
An irreversible heat engine extracts heat from a high temperature source at a rate of 100 kW and rejects heat to a sink at a rate of 50 kW. The entire work output of the heat engine is used to drive a reversible heat pump operating between a set of independent isothermal heat reservoirs at 17oC and 75oC. The rate (in kW) at which the heat pump delivers heat to its high temperature sink is
An ideal gas of mass \(m\) and temperature \(T_1\) undergoes a reversible isothermal process from an initial pressure \(P_1\) to final pressure \(P_2\). The heat loss during the process is \(Q\). The entropy change \(\Delta S\) of the gas is
Atmospheric air (\(R=287\) J/kg.K; \(\gamma =1.4\)) at 1 bar and 25oC is compressed adiabatically to 2 bar and 105oC . Which of the following statements is correct?
A heat pump, which operates in a cycle, extracts heat energy from the cold reservoir and supplies the same amount of energy to the hot reservoir. Which of the following statements holds for this process?
For a reversible isothermal expansion of an ideal gas from a state 1 to a state 2,
A process is irreversible as long as entropy change (\(\Delta S\)) for the system is greater than zero. (True/False)
\(10^6\) Joules of heat are transferred from a reservoir at 327\(^\circ \)C to an engine that operates on the Carnot cycle. The engine rejects heat to a reservoir at 27\(^\circ \)C. Determine: (i) the thermal efficiency of the cycle (%), and (ii) the work done (in MJ) by the engine.
(i) {#1}
(ii) {#2}
A solid metallic block weighing 5 kg has an initial temperature of 500oC; 40 kg of water initially at 25oC is contained in a perfectly insulated tank. The metallic block is brought into contact with water. Both of them come to equilibrium. Specific heat of block material is 0.4 kJ kg-1.K-1. Ignoring the effect of expansion and contraction, and also the heat capacity of tank, the total entropy change in kJ.K-1 is
A Carnot heat engine cycle is working with an ideal gas. The work performed by the gas during the adiabatic expansion and compression steps, \(W_1\) and \(W_2\) respectively, are related as
A heat transformer is a device that transfers a part of the heat, supplied to it at an intermediate temperature, to a high temperature reservoir while rejecting the remaining part to a low temperature heat sink. In such a heat transformer, 100 kJ of heat is supplied at 350 K. The maximum amount of heat in kJ that be transferred to 400 K, when the rest is rejected to a heat sink at 300 K is
A cyclic device operates between three thermal reservoirs, as shown in the figure. Heat is transferred to/from the cyclic device. It is assumed that heat transfer between each thermal reservoir and the cyclic device takes place across negligible temperature difference. Interactions between the cyclic device and the respective thermal reservoirs that are shown in the figure are all in the form of heat transfer.
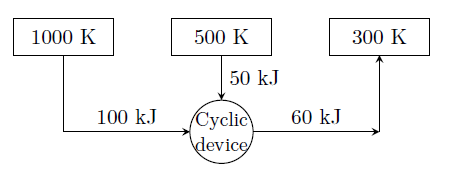
The cyclic device can be
Consider the following two processes:
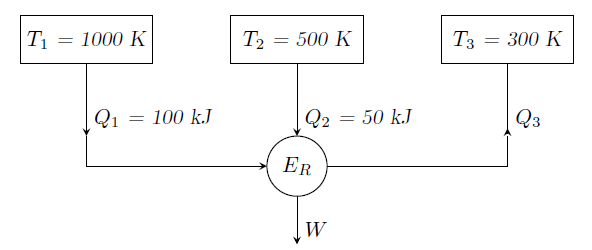
Figure below shows a reversible heat engine \(E_R\) having heat interactions with three constant temperature systems. Calculate the thermal efficiency (in %) of the heat engine.
Calculate the change in entropy (cal/g.\(^\circ \)C) when one gram of ice at 0\(^\circ \)C is converted into steam at 100\(^\circ \)C.
The latent heat of fusion of ice = 80 cal/g. Latent heat of vaporization of water = 540 cal/g and the mean specific heat of water between 0\(^\circ \)C and 100\(^\circ \)C = 1 cal/(g.\(^\circ \)C).
In a process industry, two different streams of water (to be considered incompressible) are available at 10oC and 90oC as shown in the figure. Mass flow rates of both the streams are 1 kg/s. Rather than wasting these resources, it is desired to connect a reversible Carnot engine that will continuously extract heat from the hot stream and supply part of it to the cold stream such that the exit temperatures of both the streams \(T_f\) is identical. Heat capacity of water is 4.18 kJ/(kg.K).

(i) Value of \(T_f\) is
{#1}
(ii) Work output \(\dot {W}\) is
{#2}
An inventor claims to have developed a refrigeration unit which maintains the refrigerated space at \(-3\)\(^\circ \)C while operating in a room where the temperature is 27\(^\circ \)C, and which has a coefficient of performance of 9.5. How do you evaluate his claim?
In the figure shown, the system is a pure substance kept in a piston-cylinder arrangement. The system is initially a two-phase mixture containing 1 kg of liquid and 0.03 kg of vapor at a pressure of 100 kPa. Initially, the piston rests on a set of stops, as shown in the figure. A pressure of 200 kPa is required to exactly balance the weight of the piston and the outside atmospheric pressure. Heat transfer takes place into the system until its volume increases by 50%. Heat transfer to the system occurs in such a manner that the piston, when allowed to move, does so in a very slow (quasi-static/quasi-equilibrium) process. The thermal reservoir from which heat is transferred to the system has a temperature of 400oC. Average temperature of the system boundary can be taken as 175oC. The heat transfer to the system is 1 kJ, during which its entropy increases by 10 J/K.
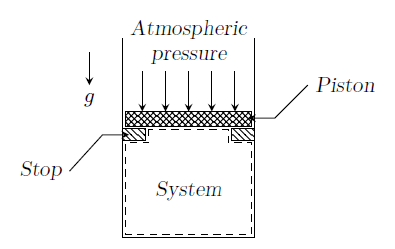
Specific volumes of liquid (\(V_L\)) and vapor (\(V_V\)) phases, as well as values of saturation temperatures, are given in the table below.
| Pressure (kPa) |
Saturation temperature \(T_{\text{sat}}\) ( \(^\circ\)C) |
\(V_L\) (m\(^3\)/kg) | \(V_V\) (m\(^3\)/kg) |
|---|---|---|---|
| 100 | 100 | 0.001 | 0.1 |
| 200 | 200 | 0.0015 | 0.002 |
(i) At the end of the process, which one of the following situations will be true?
{#1}
(ii) The work done by the system during the process is
{#2}
(iii) The net entropy generation (considering the system and the thermal reservoir together) during the process is closest to
{#3}
Last Modified on: 04-May-2024
Chemical Engineering Learning Resources - msubbu
e-mail: learn[AT]msubbu.academy
www.msubbu.in