Thermodynamics - GATE-CH Questions
Home -> GATE Questions with Solutions at MSubbu.Academy -> Thermodynamics->
Refrigeration
GATE-CH-2008-6-td-1mark
For a Carnot refrigerator operating between 40oC and 25oC , the coefficient of performance is
GATE-ME-2009-8-td-1mark
In an ideal vapor compression refrigeration cycle, the specific enthalpy of refrigerant (in kJ/kg) at the following states is given as:
| Inlet of condenser: |
283 |
| Exit of condenser: |
116 |
| Exit of evaporator: |
232 |
GATE-XE-2014-E-6-td-1mark
The efficiency of a reversible engine operating between two temperatures is 40%. The COP of a reversible refrigerator operating between the same temperatures is
GATE-CH-1987-16-ii-td-2mark
In a vapor-compression refrigeration system, designed to maintain an enclosure at \(-3^\circ \text {C}\), the equipments are so sized that all heat transfer units realize an approach of 5\(^\circ \)C. Cooling water is available at 32\(^\circ \)C. Given that:
Enthalpy of saturated refrigerant vapor = 180 kJ/kg
Enthalpy of refrigerant vapor leaving the compressor = 206 kJ/kg
Enthalpy of saturated liquid leaving the throttle valve = 61.0 kJ/kg
Estimate:
(i) the maximum possible C.O.P.
{#1}
(ii) The C.O.P. obtained in the cycle.
{#2}
Solution
GATE-ME-2009-51-52-td-4mark
The inlet and the outlet conditions of steam for an adiabatic steam turbine are as indicated in the figure. The notations are as usually followed.
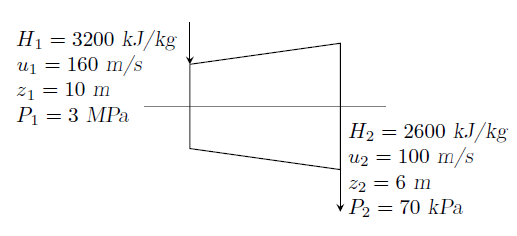
(i) If mass flow rate of steam through the turbine is 20 kg/s, the power output of the turbine (in MW) is
{#1}
(ii) Assume the above turbine to be part of a simple Rankine cycle. The density of water at the inlet to the pump is 1000 kg/m3. Ignoring kinetic and potential energy effects, the specific work (in kJ/kg) supplied to the pump is
{#2}
Solution
[Index]
GATE-PI-2010-39-td-2mark
In a steam power plant, the turbine power output is 1 MW while the boiler heat input is at the rate of 2.5 MW. The pump power input is negligibly small. In the condenser, exhaust steam from the turbine rejects heat to a steady flow of cooling water, which enters the condenser at 25oC and leaves at 40oC. Ignore kinetic and potential energy effects for the cooling water. The specific heat of cooling water is 4 kJ/kg.K. The required mass flow rate (in kg/s) of cooling water is
GATE-CH-1989-16-ii-td-4mark
An ideal Rankine cycle operates with steam at a pressure of 10 bar and temperature of 250\(^\circ \)C. The pressure in the condenser is 0.12 bar and the temperature of the condensate is 50\(^\circ \)C. The following enthalpy data are available:
Enthalpy before expansion, \(H_1\) = 2950 kJ/kg
Enthalpy after isentropic expansion, \(H_2\) = 2221 kJ/kg
Enthalpy of condensate at 0.12 bar and 50\(^\circ \)C, \(H_w\) = 204 kJ/kg
Show the enthalpy-entropy (\(H\)-\(S\)) diagram for the cycle.
Calculate the thermodynamic efficiency (in %) of the ideal Rankine cycle.
0200-4-td-5mark
A vapor compression refrigeration cycle using tetrafluoro ethane (R-134a) refrigerant is operating between the pressure limits of 0.17074 MPa and 1.13 MPa. The compression is isentropic with saturated vapor at the inlet, and condenser exit is a saturated liquid. The following table gives the saturation values of the refrigerant at the two pressure values. Specific heat of super-heated vapor at 1.13 MPa shall be taken as 1.15 kJ/kg.\(^\circ\)C.
| Pressure |
Temperature |
Enthalpy |
Entropy |
| (MPa) |
(\(^\circ\)C) |
(kJ/kg) |
kJ/kg.\(^\circ\)C |
|
|
Liquid |
Vapor |
Liquid |
Vapor |
| 0.17074 |
\(-14\) |
181.56 |
390.33 |
0.9311 |
1.7367 |
| 1.130 |
44 |
262.38 |
421.28 |
1.2091 |
1.7101 |
-
Calculate the COP of refrigeration if the expansion is happening with a (i) isentropic expander (ii) expansion valve.
-
For expansion with expansion valve, calculate (i) mass flow rate of refrigerant, and (ii) power required for compressor, for a refrigeration capacity of 2 ton.
[Index]
Last Modified on: 04-May-2024
Chemical Engineering Learning Resources - msubbu
e-mail: learn[AT]msubbu.academy
www.msubbu.in
