GATE-CH-1994-4-p-td-1mark
Match the following:
-
I. for any cyclic process
-
II. for any adiabatic process
GATE-CH-1991-7-ii-td-2mark
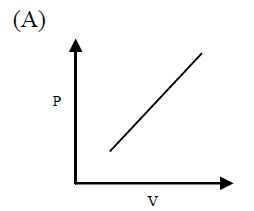
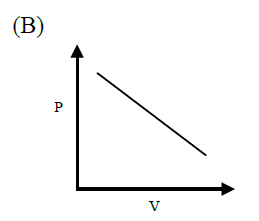
For an ideal gas the slope of the pressure-volume curve at a given point, will be
- steeper for an isothermal than for an adiabatic process
- steeper for an adiabatic than for an isothermal process
- identical for both the processes
- of opposite signs
GATE-CH-1993-5-c-td-1mark
The first law of thermodynamics takes the form \(W = \Delta H\) when applied to:
- A closed system undergoing a reversible adiabatic process
- An open system undergoing an adiabatic process with negligible changes in kinetic and potential energies
- A closed system undergoing a reversible constant volume process
- A closed system undergoing a reversible constant pressure process
GATE-CH-1998-2-17-td-2mark
It is desired to bring about a certain change in the state of a system by performing work on the system under adiabatic conditions.
- The amount of work needed is path-dependent
- Work alone cannot bring about such a change of state
- The amount of work needed is independent of path
- More information is needed to conclude anything about the path dependence or otherwise the work needed
GATE-CH-2001-2-7-td-2mark
Air enters an adiabatic compressor at 300 K. The exit temperature for a compression ratio of 3, assuming air to be an ideal gas (\(\gamma = C_P/C_V = 7/5\)) and the process to be reversible, is
- \(300(3^{2/7})\)
- \(300(3^{3/5})\)
- \(300(3^{3/7})\)
- \(300(3^{5/7})\)
GATE-CH-2003-7-td-1mark
In Joule's experiments, an insulated container contains 20 kg of water initially at 25oC . It is stirred by an agitator, which is made to turn by a slowly falling body weighing 40 kg through a height of 4 m. The process is repeated 500 times. The acceleration due to gravity is 9.8 m/sec2. Neglecting the heat capacity of agitator, the temperature of water (in oC ) is
- 40.5
- 34.4
- 26.8
- 25
GATE-CH-2004-44-td-2mark
A car tyre of volume 0.057 m3 is inflated to 300 kPa at 300 K. After the car is driven for ten hours, the pressure in the tyre increases to 330 kPa. Assume that air is an ideal gas and \(C_V\) for air is 21 J/(mol.K). The change in internal energy of air in the tyre in J/mol is
- 380
- 630
- 760
- 880
GATE-CH-2007-30-td-2mark
For the two paths as shown in the figure, one reversible and one irreversible, to change the state of the system from \(A\) to \(B\),
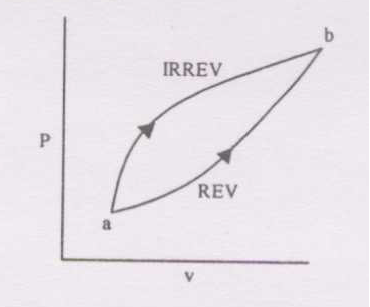
- \(\Delta U,Q,W\) are same
- \(\Delta U\) is same
- \(Q,W\) are same
- \(\Delta U, Q\) are different
GATE-CH-2007-6-td-1mark
The state of an ideal gas is changed from \((T_1,P_1)\) to \((T_2,P_2)\) in a constant volume process. To calculate the change in enthalpy, \(\Delta H\), ALL of the following properties/variables are required.
- \(C_V, P_1, P_2\)
- \(C_P, T_1, T_2\)
- \(C_P, T_1, T_2, P_1, P_2\)
- \(C_V, P_1, P_2, T_1, T_2\)
GATE-CH-2011-14-td-1mark
One mole of methane is contained in a leak proof piston-cylinder assembly at 8 bar and 1000 K. The gas undergoes isothermal expansion to 4 bar under reversible conditions. Methane can be considered as an ideal gas under these conditions. The value of the universal gas constant is 8.314 J.mol-1.K-1. The heat transferred (in kJ) during the process is
- 11.52
- 5.76
- 4.15
- 2.38
GATE-CH-2013-6-td-1mark
The thermodynamic state of a closed system containing a pure fluid changes from \((T_1, P_1)\) to \((T_2, P_2)\), where \(T\) and \(P\) denote the temperature and pressure, respectively. Let \(Q\) denote the heat absorbed (\(> 0\) if absorbed by the system) and \(W\) the work done (\(> 0\) if done by the system). Neglect changes in kinetic and potential energies. Which one of the following is CORRECT?
- \(Q\) is path-independent and \(W\) is path-dependent
- \(Q\) is path-dependent and \(W\) is path-independent
- \((Q - W)\) is path-independent
- \((Q + W)\) is path-independent
GATE-CH-2014-6-td-1mark
In a closed system, the isentropic expansion of an ideal gas with constant specific heats is represented by
GATE-CH-1993-6-2-td-1mark
A vertical cylinder with a freely floating piston contains 0.1 kg air at 1.2 bar and a small electric resistor. The resistor is wired to an external 12 Volt battery. When a current of 1.5 Amps is passed through the resistor for 90 sec, the piston sweeps a volume of 0.01 m\(^3\). Assume (i) piston and the cylinder are insulated and (ii) air behaves as an ideal gas with \(C_V = 700\) J/(kg.K). Find the rise in temperature (in \(^\circ \)C) of air.
GATE-XE-2007-G-22-td-2mark
Consider steady flow of air (\(C_P=1005\) J/kg.K) in an adiabatic passage. Air enters the passage at 100 kPa, 500 K at a velocity of 150 m/s and exits the passage at 510 K. Assume air to be an ideal gas and neglect gravitational effects. The passage is a
- diffuser, and the velocity at the exit is approximately 49 m/s
- diffuser, and the velocity at the exit is approximately 79 m/s
- nozzle, and the velocity at the exit is approximately 179 m/s
- nozzle, and the velocity at the exit is approximately 249 m/s
GATE-XE-2010-E-12-td-2mark
Air (\(R=287\) J.kg-1.K-1, \(C_P=1005\) J.kg-1.K-1 and \(\gamma =1.4\)) flows sequentially through a compressor, a heater and a turbine as shown in the figure. Volume flow rate of air coming out from the compressor is 2.33 m3/s when pressure and temperature are 276 kPa and 43oC respectively. Air is then heated at same pressure to 430oC in a heater. From heater, air flows through a turbine which produces 1860 kW of power. Heat loss from turbine to the surrounding is 90 kW. Air temperature at the turbine exit is ________________
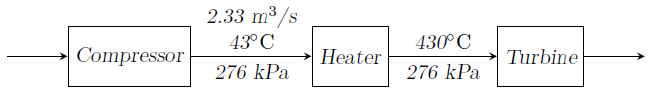
- 156.4oC
- 181.6oC
- 223.7oC
- 678.4oC
GATE-CH-2016-32-td-2mark
An ideal gas is adiabatically and irreversibly compressed from 3 bar and 300 K to 6 bar in a closed system. The work required for the irreversible compression is 1.5 times the work that is required for reversible compression from the same initial temperature and pressure to the same final pressure. The molar heat capacity of the gas at constant volume is 30 J.mol\(^{-1}\).K\(^{-1}\) (assumed to be independent of temperature); universal gas constant, \(R\) is 8.314 J.mol\(^{-1}.K^{-1}\); ratio of molar heat capacities is 1.277. The temperature (in K, rounded off to the first decimal place) of the gas at the final state in the irreversible compression case is ____________
GATE-CH-2005-81-td-4mark
A frictionless cylinder piston assembly contains an ideal gas. Initially, pressure (\(P_1\)) = 100 kPa, temperature (\(T_1\)) = 500 K and volume (\(V^{\text {t}}_1\)) = \(700 \times 10^{-6}\) m3. This system is supplied with 100 J of heat and pressure is maintained constant at 100 kPa. The enthalpy variation is given by \[ H \text { (J/mol)} = 30,000 + 50\; T \] where \(T\) is the temperature in K, and the universal gas constant \(R\) = 8.314 J/(mol.K).
(i) The final volume of the gas (\(V^{\text {t}}_2\)) in m3 is
{#1}
(ii) The change in internal energy of the gas in J is
{#2}
GATE-CH-2007-82-83-td-4mark
A perfectly insulated cylinder of volume 0.6 m3 is initially divided into two parts by a thin, frictionless piston, as shown in the figure. The smaller part of volume 0.2 m3 has ideal gas at 6 bar pressure and 100oC. The other part is evacuated.
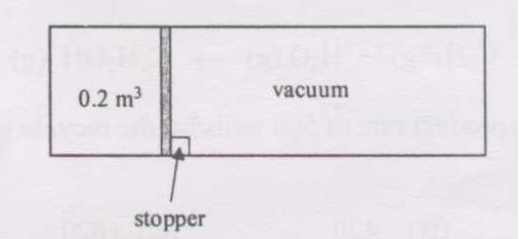
(i) At certain instant of time \(t\), the stopper is removed and the piston moves out freely to the other end. The final temperature is
{#1}
(ii) The cylinder insulation is now removed and the piston is pushed back to restore the system to its initial state. If this is to be achieved only by doing work on the system (no heat addition, only heat removal allowed), what is the minimum work required ?
{#2}
GATE-CH-2009-51-52-td-4mark
An ideal gas with molar heat capacity \(C_P=5R/2\) (where \(R=8.314\) J.mol-1.K-1) is compressed adiabatically from 1 bar and 300 K to pressure \(P_2\) in a closed system. The final temperature after compression is 600 K and the mechanical efficiency of compression is 50%.
(i) The work required for compression (in kJ/mol) is
{#1}
(ii) The final pressure \(P_2\) (in bar) is
{#2}
GATE-XE-2009-E-23-24-td-4mark
An insulated vertical cylinder encloses 0.1 kg of argon (Ar) with the help of a frictionless non-conducting piston as shown in the figure. The mass of the piston is 5 kg and it initially rests on the bottom of the cylinder. The cylinder is connected to a nitrogen (N2) tank at 100 bar through a pipeline fitted with a valve. The valve is opened and nitrogen is slowly admitted into the cylinder. During this operation, the piston is lifted through a height of 10 cm by the nitrogen gas . The initial pressure and temperature of argon gas are 100 kPa and 300 K respectively. The final temperature of argon is 320 K. For argon, \(C_P=520\) J/kg.K and \(C_V=312\) J/kg.K.
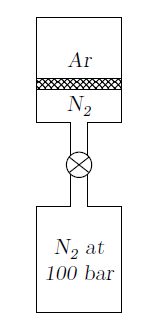
(i) The work done by argon in kJ during the process is
{#1}
(ii) The work done by nitrogen in kJ during the process is
{#2}
GATE-XE-2011-E-21-22-td-4mark
An ideal gas undergoes a cyclic process consisting of the following three processes:
Process 1 - 2: Compression process with \(PV= \text {constant}\)
Process 2 - 3: Constant pressure
Process 3 - 1: Constant volume; \(U_3-U_1=3549\) kJ
Changes in kinetic and potential energies are neglected.
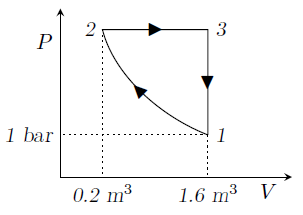
(i) The work done (kJ) during the process 2 - 3 is
{#1}
(ii) The heat transferred (kJ) during the process 2 - 3 is
{#2}
GATE-CH-2001-6-td-5mark
100 m\(^3\) of carbon dioxide initially at 423 K and 50 bar (\(50\times10^5\) Pa) is to be isothermally compressed in a frictionless piston and cylinder device to a final pressure of 300 bar (\(300\times10^5\) Pa). Assuming ideal gas behavior (\(R = 8.314 \times10^{-2}\) bar.m\(^3\)/kmol.K),
-
Write a general expression for the energy balance for the gas within the piston and cylinder device as the system, and define all the terms.
-
Calculate the volume of the compressed carbon dioxide gas at 300 bar.
-
Calculate the work done to compress the carbon dioxide gas.
-
Calculate the heat flow on compression.
Last Modified on: 04-May-2024
Chemical Engineering Learning Resources - msubbu
e-mail: learn[AT]msubbu.academy
www.msubbu.in