GATE-CH-1997-2-5-pc-2mark
1997-2-5-pc
The flowsheet is given in figure.
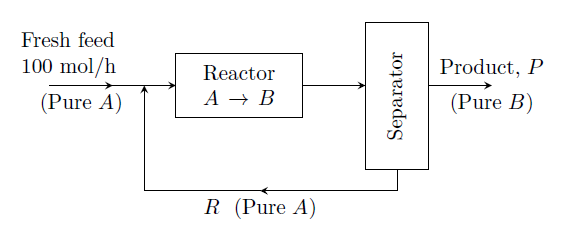
If the single-pass conversion (once-through conversion) of ¥(A¥) to ¥(B¥) is 20%, then the rate of recycle ¥(R¥) (mol/h) is
- 300
- 400
- 500
- 600
1988-1-i-pc
Match the following:
I. A part of the product stream is sent back for reprocessing
II. A part of the reactant stream that does not undergo the processing but joins the product stream
III. The entire stream or a part of it is let out of the processing zone due to build-up of non-reacting components beyond a permissible level in it
IV. The process parameters change with time
1993-8-b-pc
Methane is completely burnt with air. The maximum possible volume percent of carbon dioxide (on dry basis) in the flue gas is
1995-2-h-pc
Pure ¥(¥ce {O2}¥) is mixed with air to produce an enriched air containing 50 volume % of ¥(¥ce {O2}¥). The ratio of moles of air to ¥(¥ce {O2}¥) used is
1998-1-8-pc
Pure ethanol vapor is fed to a reactor packed with Alumina catalysts at the rate of 100 kmol/h. The reactor product comprise: Ethylene = 95 kmol/h, Water vapor = 97.5 kmol/h and diethyl ether = 2.5 kmol/h.
The reactions occurring can be represented by: ¥[ ¥begin {align*} ¥ce {C2H5OH} &¥rightarrow ¥ce {C2H4} + ¥ce {H2O} ¥¥
2 ¥ce {C2H5OH} &¥rightarrow ¥ce {C2H5-O-C2H5} + ¥ce {H2O} ¥end {align*}¥] The percent conversion of ethanol in the reactor is:
1999-1-6-pc
Pure ¥(A¥) in gas phase enters a reactor. 50% of this ¥(A¥) is converted to ¥(B¥) through the reaction ¥(A ¥rightarrow 3B¥). Mole fraction of ¥(A¥) in the exit stream is
1988-1-ii-a-pc
Carbon is burnt with air. Maximum possible volume percent ¥(¥ce {CO2}¥) in the flue gas is 末末- %
1992-2-b-pc
1.2 gatoms of carbon and 1.5 gmol of oxygen are reacted to give 1 gmol of carbon dioxide. (i) The limiting reactant is 末末- . (ii) The percent excess reactant supplied is 末末-
(i)
{#1}
(ii)
{#2}
1995-6-pc
Methanol is produced by the reaction of ¥(¥ce {CO}¥) with ¥(¥ce {H2}¥) as ¥[ ¥ce {CO} + 2¥ce {H2} ¥rightarrow ¥ce {CH3OH} ¥] Only 15% of carbon monoxide entering the reactor is converted to methanol. The methanol formed is condensed and recovered completely. The unreacted ¥(¥ce {CO}¥) and ¥(¥ce {H2}¥) are recycled back to the reactor. The fresh feed will contain ¥(¥ce {H2}¥) and ¥(¥ce {CO}¥) in the molar ratio of 2:1. For 3200 kg/h of methanol produced, calculate:
(i) kmol/h of fresh feed
{#1}
(ii) kmol/h of recycle gas
{#2}
1996-12-pc
Methanol vapor can be converted into formaldehyde by the following reaction scheme: ¥[ ¥begin {align*} ¥ce {CH3OH} + ¥ce {1/2 O2} &¥rightarrow ¥ce {HCHO} + ¥ce {H2O} ¥¥
¥ce {CH3OH} &¥rightarrow ¥ce {HCHO} + ¥ce {H2} ¥end {align*} ¥] The fresh feed to the process was 0.5 kmol/h of ¥(¥ce {O2}¥) and an excess methanol. All of the ¥(¥ce {O2}¥) reacts in the reactor. Formaldehyde and water are removed from the product stream first, after which ¥(¥ce {H2}¥) is removed from the recycled methanol. The recycle flow rate of methanol was 1 kmol/h. The ratio of methanol reacting by decomposition to that by oxidation was 3. Draw the flow diagram and then calculate: (i) the per pass conversion of methanol in the reactor, and, (ii) the fresh feed rate of methanol (kmol/h).
(i) ____________
{#1}
(ii) ____________
{#2}
1997-2-4-pc
Pure carbon is completely burnt in oxygen. The flue gas analysis is 70% ¥(¥ce {CO2}¥), 20% ¥(¥ce {CO}¥) and 10% ¥(¥ce {O2}¥). The percent excess oxygen used is
1997-2-5-pc
The flowsheet is given in figure.

If the single-pass conversion (once-through conversion) of ¥(A¥) to ¥(B¥) is 20%, then the rate of recycle ¥(R¥) (mol/h) is
2001-2-3-pc
2003-38-pc
2005-43-pc
A feed stream (¥(S_1¥)) at 100 kg/h and containing only ¥(A¥) mixes with recycle stream ¥(S_5¥) before entering the reactor (see the figure below), where the reaction ¥(A¥rightarrow B¥) takes place. The operation is at steady state. The stream ¥(S_3¥) leaving the reactor is separated, without either phase or composition change, into two streams ¥(S_4¥) and ¥(S_5¥). If the mass fraction of ¥(B¥) in ¥(S_4¥) is 0.95 and total flow rate of ¥(S_5¥) is 10 kg/h, then the ratio of flow rates of streams ¥(S_3/S_5)¥), and the flow rate of ¥(A¥) in ¥(S_3¥) are, respectively,

2008-31-pc
Air (79 mole% nitrogen and 21 mole% oxygen) is passed over a catalyst at high temperature. Oxygen completely reacts with nitrogen as shown below ¥[ ¥begin{align*}0.5 ¥text{N}_2¥text{(g)} + 0.5 ¥text{O}_2¥text{(g)} &¥rightarrow ¥text{NO}¥text{(g)} ¥¥ 0.5 ¥text{N}_2¥text{(g)} + ¥text{O}_2¥text{(g)} &¥rightarrow ¥text{NO}_2¥text{(g)}¥end{align*} ¥] The molar ratio of NO to NO2 in the product stream is 2:1. The fractional conversion of nitrogen is
2008-34-pc
Carbon black is produced by decomposition of methane: ¥[ ¥text{CH}_4¥text{(g)} ¥rightarrow ¥text{C}¥text{(s)} + 2¥text{H}_2¥text{(g)} ¥] The single pass conversion of methane is 60%. If fresh feed is pure methane and 25% of the methane exiting the reactor is recycled, then the molar ratio of fresh feed stream to recycle stream is
2011-31-pc
Ammonia is synthesised at 200 bar and 773 K by the reaction ¥( ¥text{N}_2 + 3¥text{H}_2 ¥rightleftharpoons 2¥text{NH}_3¥). The yield of ammonia is 0.45 mol/mol of fresh feed. Flow sheet for the process (along with available compositions) is shown below.

The single-pass conversion for H2 in the reactor is 20%. The amount of H2 lost in the purge as a PERCENTAGE of H2 in fresh feed is
1989-1-iii-pc
In a reaction mixture the reactants ¥(¥ce {CO}¥) and ¥(¥ce {O2}¥) are present in the mole ratio of 1:0.4. Starting with a total of 1.4 moles of the reactants, the products obtained after oxidation of ¥(¥ce {CO}¥) to ¥(¥ce {CO2}¥) contain: ¥(¥ce {CO2}¥) = 0.6 moles, ¥(¥ce {CO}¥) = 0.4 moles, ¥(¥ce {O2}¥) = 0.1 moles. The degree of completion of the oxidation reaction is 末末- %.
1989-11-ii-pc
The gases entering a reactor contain ¥(A¥) and ¥(B¥) in the mole ratio of 1:4. The mole ratio of these gases in the exit stream from the reactor is 1:4.25. What volume of the gas in m¥(^3¥) at STP must enter the reactor to produce 100 kmol of product ¥(C¥) per hour?
The reaction is ¥(A + 3B ¥rightarrow 2C¥).
1990-11-ii-pc
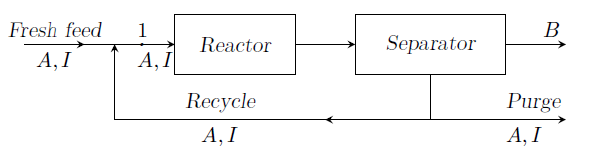
For the reaction ¥(A¥rightarrow B¥), the process flow diagram is shown in figure. The fresh feed of ¥(A¥) consists of 0.5% of inerts by volume. 60% conversion per pass of ¥(A¥) fed to the reactor is obtained. The concentration of inerts going into the reactor at (1) must be held at 2% by volume. All streams are ideal gases and the process is at steady state. How many moles need to be recycled per mole of total feed to the reactor at (1).
1991-12-ii-pc
Limestone mixed with coke is being burnt in a kiln. An average analysis of limestone is ¥(¥ce {CaCO3}¥): 84.5%, ¥(¥ce {MgCO3}¥): 11.5% and the rest inerts. The coke contains 76% carbon, 21% ash and 3% moisture. The calcination of ¥(¥ce {CaCO3}¥) is only 95% complete and that of ¥(¥ce {MgCO3}¥) 90%. The carbon in the coke is completely burnt to ¥(¥ce {CO2}¥). The kiln is fed with 1 kg of coke per 5 kg limestone. Calculate weight percent ¥(¥ce {CaO}¥) in the product leaving the kiln.
1992-12-a-pc
The analysis of the gas entering the secondary converter in a contact sulphuric acid plant is 4% ¥(¥ce {SO2}¥), 13% ¥(¥ce {O2}¥) and 83% ¥(¥ce {N2}¥) (volume %). In the converter ¥(¥ce {SO2}¥) is oxidized to ¥(¥ce {SO3}¥). The gases leaving the converter contain 0.45% ¥(¥ce {SO2}¥) on an ¥(¥ce {SO3}¥) - free basis (volume %). Calculate the percent conversion of ¥(¥ce {SO2}¥).
2014-41-pc
1988-11-i-pc
Exit gases from an ethylene oxide reactor had the following analysis (mol% on dry basis): ethylene 2.3, ethylene oxide 0.9, nitrogen 79.0, oxygen 12.3 and carbon dioxide 5.5. Calculate:
(a) percent selectivity
{#1}
(b) percent overall conversion
{#2}
(c) moles of air per mole of ethylene in the feed mixture.
{#3}
1993-18-pc
Iron pyrites (¥(¥ce {FeS2}¥)) is burned with air in 100% excess of that required to oxidise all iron to ¥(¥ce {Fe2O3}¥) and all sulphur to sulphur dioxide. Calculate the composition (in mol%) of exit gases, if 80% of sulphur is oxidised to sulphur trioxide and the rest to sulphur dioxide. All iron is oxidised to ¥(¥ce {Fe2O3}¥).
(i) ¥(¥ce {SO2}¥)
{#1}
(ii) ¥(¥ce {SO3}¥)
{#2}
(iii) ¥(¥ce {O2}¥)
{#3}
(iv) ¥(¥ce {N2}¥)
{#4}
2007-78-79-pc
A simplified flowsheet is shown in the figure for production of ethanol from ethylene. The conversion of ethylene in the reactor is 30% and the scrubber following the reactor completely separates ethylene (as top stream) and ethanol and water as bottoms. The last (distillation) column gives an ethanol-water azeotrope (90 mol% ethanol) as the final product and water as waste. The recycle to purge ratio is 34.
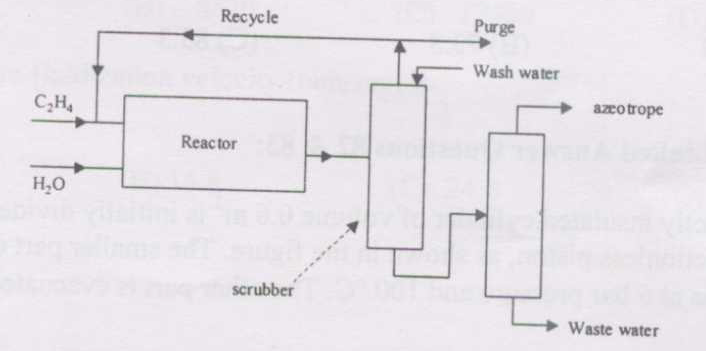
(i) For an azeotrope product rate of 500 mol/h, the recycle gas flowrate in mol/h is
{#1}
(ii) For the same process, if fresh H2O feed to the reactor is 600 mol/h and wash water for scrubbing is 20% of the condensables coming out of the reactor, the water flowrate in mol/h from the distillation column as bottoms is
{#2}
1998-3-pc
Ethylene oxide is produced by the oxidation of ethylene over a catalyst. Safety considerations dictate that the gaseous mixture entering the reactor should contain 10 mol air per mol ethylene. The conversion per pass is 22%. The ethylene oxide formed is completely condensed out and the remaining gases recycled. Make up oxygen is added to maintain the request oxygen levels. For a plant producing 440 kg/h of ethylene oxide,
Calculate the quantity of pure makeup oxygen to be supplied, in kg/h, in steady state operation.
Draw a neat block diagram showing the major units, flows and compositions, and indicate the envelope / boundary around which the requisite mass balance(s) is / are made.
The relevant reaction is represented by ¥[2¥ce{C2H4(g)} + ¥ce{O2(g)} ¥rightarrow 2¥ce{C2H4O(g)}¥] [Atomic masses as: C = 12, O = 16, H = 1].
1999-6-pc
It is proposed to produce acetaldehyde by oxidation of ethanol in gas phase ¥[ ¥ce {C2H5OH + 1/2 O2(g)} ¥rightarrow ¥ce {CH3CHO(g)} + ¥ce {H2O(g)} ¥] The ratio of air to ethanol in fresh feed (before it is mixed with recycle stream) is 10 to 1. The conversion of ethanol on a single pass through reactor is 25%. The unreacted ethanol is completely separated from the reaction products and recycled. What is the ratio of recycle stream to the fresh feed stream? What is the composition of the outlet stream from the reactor in mass fraction and mole fraction?
2001-5-pc
The process schematic of a propane dehydrogenation plant is shown below. It is desired to set up a simplified version of the material balance for this plant. Assume that the only reaction is the dehydrogenation of propane to propylene; there are no side reactions. The yield of propylene per pass is 30% (i.e., 30% of propane entering the reactor is converted to propylene). Assume that the amount of carbon formed on the catalyst is negligible. The product flow rate (stream ¥(S_5¥)) is 50 kmol/h. Calculate the flow rates of all the other streams. Notice that all streams except stream ¥(S_3¥) are pure.

Last Modified on: 04-May-2024
Chemical Engineering Learning Resources - msubbu
e-mail: learn[AT]msubbu.academy
www.msubbu.in