GATE-CH-2005-41-pc-2mark
2005-41-pc

- ¥(m_6=7500¥) kg/h, ¥(w_6=0.0¥)
- ¥(m_6=7050¥) kg/h, ¥(w_6=0.04255¥)
- ¥(m_6=4500¥) kg/h, ¥(w_6=0.1712¥)
- ¥(m_6=5600¥) kg/h, ¥(w_6=0.0314¥)
2001-1-5-pc
2003-4-pc
1991-2-iv-pc
The heat absorbed (in kJ/mol) for isothermal reaction (given below) at 298 K and 1 atm pressure is ––––- ¥[ ¥ce {C4H10(g)} ¥rightarrow ¥ce {C2H4(g)} + ¥ce {C2H6(g)} ¥] Standard heat of combustion, kJ/mol:
¥(¥ce {C4H10(g)}¥) = ¥(-2873.5¥); ¥(¥ce {C2H4(g)}¥) = ¥(-1411.9¥); ¥(¥ce {C2H6(g)}¥) = ¥(-1561.0¥).
1999-1-5-pc
A solution of specific gravity 1.0 consists of 35% ¥(A¥) by weight and the remaining ¥(B¥). If the specific gravity of ¥(A¥) is 0.7, the specific gravity of ¥(B¥) is
2003-39-pc
2005-41-pc

2009-28-pc
Pure water (stream ¥(W¥)) is to be obtained from a feed containing 5 wt% salt using a desalination unit as shown below.

If the overall recovery of pure water (through stream ¥(W¥)) is 0.75 kg/kg feed, then the recycle ratio (¥(R/F¥)) is
0100-5-pc
A tank contains 1000 liter of brine with 15 kg of dissolved salt. Pure water enters the tank at a rate of 10 liter per min. The solution is kept thoroughly mixed and drains from the tank at the same rate. How much salt (in kg) is in the tank, after 20 minutes?
1989-1-ii-pc
100 m¥(^3¥) of a gas mixture containing 10% ¥(A¥) and 90% ¥(B¥) is to be enriched to contain 40% ¥(A¥) by mixing with pure ¥(A¥) at constant pressure and temperature. All compositions are in volume %. The amount of pure ¥(A¥) added is ––––- m¥(^3¥).
1989-11-i-pc
A 4% solution of a salt in water is required in a continuous process. For this purpose, a part of the water is by passed through a saturator from which a 25% solution is produced. If this stream is mixed with the main water stream to produce the 4% solution, what percentage of water must be by passed?
1992-11-pc
The concentration of ¥(¥ce {SO2}¥) in the flue gases from a boiler was found to be 0.2 kg/m¥(^3¥) at N.T.P. Determine the concentration of ¥(¥ce {SO2}¥) in parts per million by volume, at N.T.P. Assume that the flue gases are perfect.
1997-12-pc
Sea water is desalinated by reverse osmosis as shown in figure.
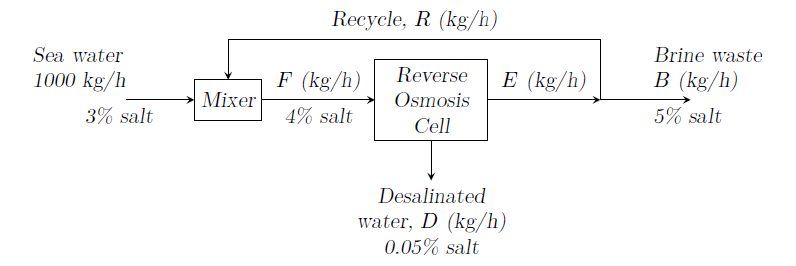
All compositions are on mass basis. Calculate ¥(R/E¥).
2013-48-49-pc
A reverse osmosis unit treats feed water ¥((F)¥) containing fluoride and its output consists of a permeate stream ¥((P)¥) and a reject stream ¥((R)¥). Let ¥(C_F, C_P¥), and ¥(C_R¥) denote the fluoride concentrations in the feed, permeate, and reject streams, respectively. Under steady state conditions, the volumetric flow rate of the reject is 60% of the volumetric flow rate of the inlet stream, and ¥(C_F = 2¥) mg/L and ¥(C_P = 0.1¥) mg/L.
(i) The value of ¥(C_R¥) in mg/L, up to one digit after the decimal point, is ____________
{#1}
(ii) A fraction ¥(f¥) of the feed is bypassed and mixed with the permeate to obtain treated water having a fluoride concentration of 1 mg/L. Here also the flow rate of the reject stream is 60% of the flow rate entering the reverse osmosis unit (after the bypass). The value of ¥(f¥), up to 2 digits after the decimal point, is ____________
{#2}
Last Modified on: 04-May-2024
Chemical Engineering Learning Resources - msubbu
e-mail: learn[AT]msubbu.academy
www.msubbu.in