Mass Transfer - GATE-CH Questions
Home -> GATE Questions with Solutions at MSubbu.Academy -> Mass Transfer->
Distillation
0600-4-mt-1mark
In steam distillation of nitrobenzene (bp. 210.6oC) at a total pressure of one atmosphere, the boiling point of mixture is
0600-5-mt-1mark
In a particular distillation operation, the feed is to be changed from saturated vapors to saturated liquid (composition unchanged), the number of plates required in enriching section would ________
Note: Distillate and residue compositions remain unchanged.
GATE-CH-1988-5-b-i-mt-1mark
Starting at the minimum reflux ratio, as the reflux ratio is increased for a given separation, the fixed cost of a fractionating column
GATE-CH-1988-6-b-i-mt-1mark
At the boiling point of a completely immiscible system of two liquids, the partial pressure of one component in the vapor phase is
GATE-CH-1989-5-ii-c-mt-1mark
By increasing the feed rate to a fractionating column, separating a binary mixture at a fixed reflux ratio and separation, the required number of ideal stages:
[Index]
GATE-CH-1989-5-ii-d-mt-2mark
By increasing the reflux ratio in a binary fractionating column from minimum reflux ratio onwards, the:
GATE-CH-1990-5-iii-mt-2mark
Minimum number of ideal stages are required in a fractionating column when the reflux ratio is equal to:
GATE-CH-1993-12-a-ii-mt-1mark
In distillation under minimum reflux conditions, number of theoretical stages would be
GATE-CH-1995-2-n-mt-2mark
In distillation where \(q\) is defined as the moles of liquid flow in the stripping section per mole of feed introduced, for saturated liquid feed
GATE-CH-1996-1-13-mt-1mark
When a multistage tray tower uses a minimum reflux ratio it implies
[Index]
GATE-CH-1997-1-14-mt-1mark
In binary distillation, the separation of the components is easier if the relative volatility(\(\alpha \)) is
GATE-CH-1997-2-12-mt-2mark
In a binary distillation column, if the feed contains 40 mol% vapor, the \(q\) line will have a slope of
GATE-CH-1998-1-15-mt-1mark
For a fixed number of ideal stages in a distillation column, as the reflux ratio is increased, the difference in composition between the product streams
GATE-CH-1998-1-18-mt-1mark
In distillation column design, the McCabe-Thiele procedure is inadequate and Ponchon-Savarit procedure is needed when
GATE-CH-1999-1-17-mt-1mark
Solvent used in extractive distillation
[Index]
GATE-CH-1999-2-12-mt-2mark
The vapor pressures of benzene and toluene are 3 and 4/3 atmospheres respectively. A liquid feed of 0.4 moles of benzene and 0.6 moles of toluene is vaporized. Assuming that the products are in equilibrium, the vapor phase mole fraction of benzene is
GATE-CH-2000-1-05-mt-1mark
Assume that benzene is insoluble in water. The normal boiling points of benzene and water are 80.1oC and 100oC respectively. At a pressure of 1 atm, the boiling point of a mixture of benzene and water is
GATE-CH-2003-18-mt-1mark
Minimum reflux ratio in a distillation column results in
GATE-CH-2004-5-mt-1mark
A distillation column separates 10,000 kg/h of a benzene-toluene mixture as shown in the figure below. In the figure, \(x_F, x_D\), and \(x_W\) represent the weight fraction of benzene in the feed, distillate, and residue, respectively.
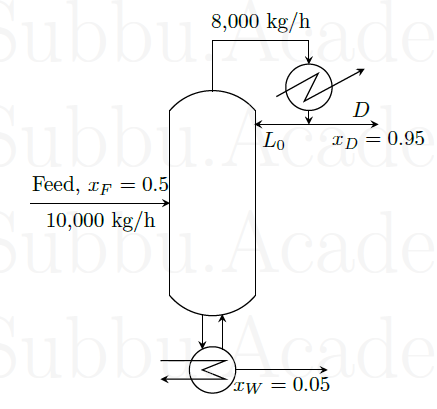
The reflux ratio is
GATE-CH-2004-69-mt-2mark
A distillation column with \(N\) plates is being operated under normal conditions. At some point in time, the operation is shifted to total reflux condition (i.e., no product and residue are being withdrawn and feed to the column is stopped). At the new steady state,
[Index]
GATE-CH-2004-70-mt-2mark
An aqueous solution of methanol is to be distilled in a tray column. High-pressure steam is available as a source of heat. For a given reflux ratio and overhead composition, two options are being explored: (i) a reboiler is used, and (ii) no reboiler is used but steam is fed directly to the bottom of the column. As compared to option (i), in option (ii):
GATE-CH-2005-4-mt-1mark
A distillation column at a pilot plant is scaled up by 3 times for industrial use at steady state. After scaling up
GATE-CH-2005-62-mt-2mark
For a two-phase feed, where 80% of the feed is vaporized under column conditions, the feed line slope in the McCabe-Thiele method for distillation column design, is
GATE-CH-2005-63-mt-2mark
A liquid mixture of benzene and toluene is in equilibrium with its vapour at 101 kPa and 373 K. The vapor pressures of benzene and toluene at 373 K respectively are 156 and 63 kPa respectively. Assuming that the system obeys Raoult’s law, the mole fraction of benzene in the liquid phase is
GATE-CH-2008-48-mt-2mark
In a binary mixture containing components \(A\) and \(B\), the relative volatility of \(A\) with respect to \(B\) is 2.5 when mole fractions are used. The molecular weights of \(A\) and \(B\) are 78 and 92 respectively. If the compositions are however expressed in mass fractions the relative volatility will then be
[Index]
GATE-CH-2012-17-mt-1mark
In McCabe-Thiele diagram, if the \(x\)-coordinate of the point of intersection of the \(q\)-line and the vapor-liquid equilibrium curve is greater than the \(x\)-coordinate of the feed point, the quality of the feed is
GATE-CH-2015-22-mt-1mark
Identify the WRONG statement amongst the following:
GATE-CH-1990-15-i-mt-6mark
A continuous rectification column is used to separate a binary mixture of \(A\) and \(B\). Distillate is produced at 100 kmol/h containing 98 mole % \(A\). The mole fractions of \(A\) in the liquid and in the vapor, \(x\) and \(y\) respectively, from two adjacent ideal plates in the enriching section are as follows:
| \(x\) |
\(y\) |
| 0.65 |
0.82 |
| 0.56 |
0.76 |
If the latent heat of vaporization is the same for all mixtures and if the feed is a saturated liquid, calculate:
(a) the reflux ratio
{#1}
(b) vapor rate in the stripping section in kmol/h.
{#2}
GATE-CH-1992-16-b-mt-6mark
The following information is available from the record of a binary fractionating column:
Feed = 180 kmol/h and 60% vaporised;
Distillate = 100 kmol/h with 0.98 mole fraction of more volatile component;
Reboiler steam demand = 420 kg/h;
Latent heat of column liquid = \(3 \times 10^4\) J/mol; and
Latent heat of steam used in reboiler = 2200 J/g
Calculate:
(a) the operating reflux ratio.
{#1}
(b) the mole fraction of more volatile component in the vapor entering that plate from which liquid overflow contains 0.7 mole fraction of the more volatile component.
{#2}
GATE-CH-2004-62-63-mt-4mark
The boiling points for pure water and pure toluene are 100oC and 110.6oC, respectively. Toluene and water are completely immiscible in each other. A well-agitated equimolar mixture of toluene and water is prepared.
(i) The temperature at which the above mixture will exert a pressure of one standard atm is
{#1}
(ii) At a total pressure of one standard atm exerted by the vapours of water and toluene, the mole fraction of water \(x_w\) in the vapour phase satisfies
{#2}
[Index]
GATE-CH-2009-55-56-mt-4mark
A flash distillation drum (see figure below) is used to separate a methanol-water mixture. The mole fraction of methanol in the feed is 0.5, and the feed flow rate is 1000 kmol/h. The feed is preheated in a heater with heat duty \(Q_h\) and is subsequently flashed in the drum. The flash drum can be assumed to be an equilibrium stage, operating adiabatically. The equilibrium relation between the mole fractions of methanol in the vapor and liquid phases is \(y^* = 4 x\). The ratio of distillate to feed flow rate is 0.5.
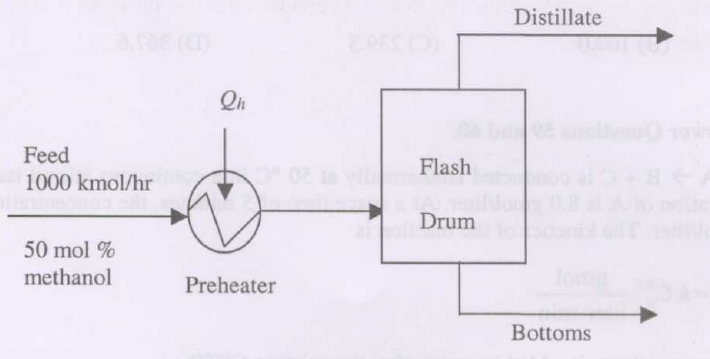
(i) The mole fraction of methanol in the distillate is
{#1}
(ii) If the enthalpy of the distillate with reference to the feed is 3000 kJ/kmol, and the enthalpy of the bottoms with reference to the feed is \(-1000\) kJ/kmol, the heat duty of the preheater (\(Q_h\) in kJ/h) is
{#2}
GATE-CH-2011-50-51-mt-4mark
A binary feed mixture containing equimolar quantities of components \(S\) and \(T\) is to be distilled in a fractionating tower at atmospheric pressure. The distillate contains 96 mole% \(S\). The \(q\)-line (feed line) intersects the equilibrium line at \(x’=0.46\) and \(y’=0.66\), where \(x’\) and \(y’\) are mole fractions. Assume that the McCabe-Thiele method is applicable and the relative volatility is constant.
(i) The minimum reflux ratio is
{#1}
(ii) The feed is
{#2}
GATE-CH-2013-52-53-mt-4mark
The vapor liquid equilibrium relation for an ideal binary system is given by \[ y_A^* = \frac {\alpha _{AB}x_A}{1+(\alpha _{AB}-1)x_A} \] Here \(x_A\) and \(y_A^*\) are the mole fractions of species \(A\) in the liquid and vapor, respectively. The relative volatility \((\alpha _{AB})\) is greater than unity.
(i) The liquid mole fraction \(x_A\) at which the maximum difference between the equilibrium vapor mole fraction and liquid mole fraction occurs is
{#1}
(ii) A liquid having the composition found in the first part of the linked answer question, is flash distilled at a steady state to a final liquid mole fraction of 0.25. If \(\alpha _{AB}\) is 2.5, the fraction of the feed vaporized is
{#2}
GATE-CH-1994-22-mt-5mark
A binary distillation column is operating under the conditions given below:
Feed rate = 350 kmol/h
Overhead product rate = 150 kmol/h
Mole fraction of more volatile component in overhead product = 0.97
Mole fraction of more volatile component in bottom product = 0.02
Bottom product rate = 200 kmol/h
Reflux ratio = 3.5
In the stripping section it was found that the mole fraction of the volatile component in the vapor leaving a plate is 0.33 while its mole fraction in the liquid coming to the same plate is 0.25. Assuming constant molal counter flow, determine whether the feed is vapor, liquid or partially vaporized.
GATE-CH-2002-2-8-mt-2mark
According to the Fenske equation, what will be the minimum number of plates required in a distillation column to separate an equimolar binary mixture of components \(A\) & \(B\) into an overhead fraction containing 99 mole % \(A\) and a bottoms fraction containing 98 mole % \(B\)? [Assume that the relative volatility (\(\alpha _{AB} = 2\)) does not change appreciably in the column]
[Index]
GATE-CH-2006-48-mt-2mark
100 moles of a binary mixture \(F\) containing 60 mol% \(A\) (more volatile) and 40 mol% \(B\) is treated in a binary distillation still. After 1 hour, 70 moles of the distillate \(D\) is collected leaving behind the residue \(W\). Relative volatility \(\alpha \) is 2. The governing equation is \[ \log \frac {Fx_F}{Wx_W} = \alpha \log \frac {F(1-x_F)}{W(1-x_W)} \] The average mole fraction of \(A\) in the distillate is
GATE-CH-2008-50-mt-2mark
A batch distillation operation is carried out to separate a feed containing 100 moles of a binary mixture of \(A\) and \(B\). The mole fraction of \(A\) in the feed is 0.7. The distillation progresses until the mole fraction of \(A\) in the residue decreases to 0.6. The equilibrium curve in this composition range may be linearized to \(y^* = 0.7353 x + 0.3088\). Here \(x\) and \(y\) are the mole fractions of the more volatile component \(A\) in the liquid and vapor phases respectively. The number of moles of residue is
GATE-CH-2012-38-mt-2mark
An equimolar mixture of \(A\) and \(B\) (\(A\) being more volatile) is flash distilled continuously at a feed rate of 100 kmol/h, such that the liquid product contains 40 mol% of \(A\). If the relative volatility is 6, then the vapor product, in kmol/h, is
GATE-CH-1988-15-iii-mt-6mark
A continuous rectifying column is separating a binary mixture containing \(A\) and \(B\) (\(A\) is more volatile than \(B\)) into an overhead distillate product containing 95 mole % \(A\). The liquid overflowing from two adjacent plates in the enriching section contain 58.6 mole % and 50 mole % \(A\) respectively. The plate efficiency is 100%. Reflux is at its bubble point. Relative volatility may be taken as 2.5. Calculate the reflux ratio. (Solve analytically).
GATE-CH-1989-15-ii-mt-4mark
A binary liquid mixture containing 50 mole % of the more volatile component is fed to a heater where 40% of the feed is flash vaporized. If the liquid and vapor produced are in equilibrium, calculate the share of more volatile component in the liquid product obtained (in mole %). Relative volatility may be taken as 2.0
[Index]
GATE-CH-1990-5-vi-mt-2mark
A binary hydrocarbon liquid mixture of \(A\) and \(B\) (\(K_A = 1.5\)) containing 60 mole% \(A\) is flash vaporised. If 40% of the feed is vaporised, the mole fraction of \(A\) in the liquid product is ––––- .
GATE-CH-1998-6-mt-5mark
100 moles of an Acetonitrile-Nitromethane mixture is differentially distilled in a batch still at a pressure of 70 kPa. The feed contains 74 mole % acetonitrile. Distillation is continued till the liquid left behind in the still contains 32 mole % acetonitrile. The vapor-liquid equilibria for the system at this pressure are correlated as follows:\[ \begin {align*} y^* = 1.05x + 0.13 & \text { for } 0.3 < x < 0.52 \\ \text { and} \\
y^* = 0.77x + 0.28 & \text { for } 0.52 < x < 0.80 \end {align*} \] where \(x\) and \(y^*\) refer to the mole fractions of acetonitrile in the liquid and equilibrium vapor respectively.
Find the average composition of the distillate (in mol% of acetonitrile) collected.
GATE-CH-2014-36-mt-2mark
A binary distillation column is operating with a mixed feed containing 20 mol% vapour. If the feed quality is changed to 80 mol% vapour, the change in the slope of the \(q\)-line is ____________
GATE-CH-2016-43-mt-2mark
A binary distillation column is to be designed using McCabe Thiele method. The distillate contains 90 mol% of the more volatile component. The point of intersection of the q-line with the equilibrium curve is \((0.5, 0.7)\). The minimum reflux ratio (rounded off to the first decimal place) for this operation is ____________
GATE-CH-2017-42-mt-2mark
The vapor phase composition and relative volatilities (with respect to n-propane) on an ideal tray of a distillation column are
| Component |
Methane |
Ethane |
n-Propane |
| Mole fraction in vapor |
0.12 |
0.28 |
0.60 |
| Relative volatility |
10 |
4 |
1 |
The mole fraction of n-propane in the liquid phase, rounded to 2 decimal places, is ____________
[Index]
GATE-CH-2006-71-72-73-mt-6mark
A binary distillation column separates 100 mol/h of a feed mixture into distillate \(D\) and residue \(W\). The McCabe-Thiele diagram for this process is given below. The relative volatility for the binary system is constant at 2.4.
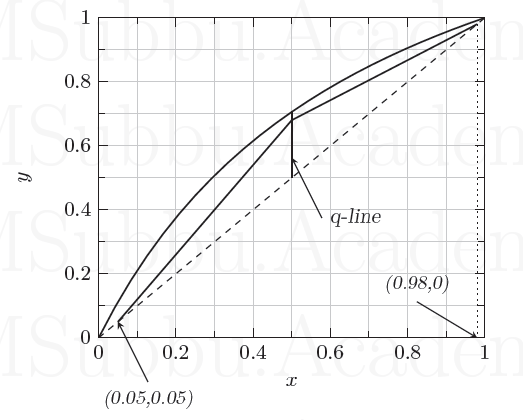
(i) The distillate and residue flow rates (in mol/h) are
{#1}
(ii) The ratio of liquid to vapor molar flow rates in the rectifying section is
{#2}
(iii) The minimum number of theoretical stages (inclusive of reboiler) for this process is
{#3}
GATE-CH-1988-5-a-iii-mt-1mark
The number of ideal stages required in a fractionating column is the least at ––––-
GATE-CH-1991-16-i-mt-8mark
A continuous stream of a binary liquid mixture, containing \(x_F\) mol fraction of \(A\), is fed into a flash distillation unit. Feed is represented by point \(Q\) on the enthalpy-concentration diagram as shown in figure. The products out of the unit are a vapor stream (\(y_V\) mol fraction of \(A\)) and a liquid stream (\(x_L\) mol fraction of \(A\)).
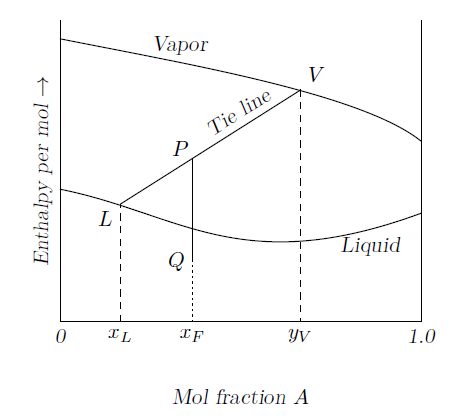
- Obtain an expression for the ratio of vapor flow rate to feed flow rate in terms of compositions.
- Show that the heat added per mole of feed is given by the distance \(PQ\).
GATE-CH-1992-6-b-mt-2mark
In the McCabe-Thiele diagram for binary distillation, vertical feed line represents ––––- feed and horizontal feed line represents ––––- feed.
GATE-CH-1997-20-mt-5mark
A feed of known composition (binary mixture of constant relative volatility) is to be distilled in a continuous fractionating column consisting of a partial condenser, one plate, and a reboiler. The feed enters the reboiler from which a bottom product is continuously withdrawn. The liquid reflux from the partial condenser is returned to the plate. The distillate composition (\(x_D\)) is 0.8 and the reflux ratio is 2.
- What is the slope of the operating line on \(x\)-\(y\) plot and its intercept on the \(y\)-axis?
- Using the McCabe-Thiele method, qualitatively locate on the \(x\)-\(y\) plot:
- the composition of the streams leaving the plate, and
- the bottom product composition.
(Do not use graph paper)
GATE-CH-2000-9-mt-5mark
Obtain the equation for the \(q\)-line given that the operating lines are: \[\begin{align*} y &= \frac{L}{L+D}x + \frac{D}{L+D}x_D \qquad \qquad & \text{ (enriching section) } \\ y &= \frac{\overline{L}}{\overline{L}-B}x - \frac{B}{\overline{L}-B}x_B & \text{ (stripping section) } \end{align*}\] where \(L\) and \(\overline{L}\) are the liquid flow rates in the enriching and stripping sections, \(D\) and \(B\) are the top and bottom product flow rates, and \(x_D\) and \(x_B\) are the mole fractions of top and bottom products respectively.
[Index]
Last Modified on: 03-May-2024
Chemical Engineering Learning Resources - msubbu
e-mail: learn[AT]msubbu.academy
www.msubbu.in



