Mass Transfer - GATE-CH Questions
Home -> GATE Questions with Solutions at MSubbu.Academy -> Mass Transfer->
Absorption
GATE-CH-1988-5-b-iii-mt-1mark
In an absorber the equilibrium curve is always
GATE-CH-1988-5-b-iv-mt-1mark
Kremser-Brown-Souders equation is used to calculate
GATE-CH-1989-5-ii-a-mt-1mark
NTU can be considered as a:
GATE-CH-1993-7-a-iii-mt-1mark
Absorption towers are operated under conditions of
GATE-CH-1997-1-13-mt-1mark
For stripping of a gas in a counter current stripper, the operating line
[Index]
GATE-CH-2000-1-14-mt-1mark
The absorption factor is defined as __________
where \(L\) = liquid flow rate, \(G\) = gas flow rate and \(m\) = slope of the equilibrium line.
GATE-CH-2008-16-mt-1mark
In a countercurrent gas absorber, both the operating and equilibrium relations are linear. The inlet liquid composition and the exit gas composition are maintained constant. In order to increase the absorption factor
GATE-CH-2009-12-mt-1mark
The ratio of the liquid to gas flow rate in a counter-current gas absorption column is increased at otherwise identical conditions. Which ONE of the following statements is TRUE ?
GATE-CH-2013-19-mt-1mark
The packing of an existing absorption tower is replaced with a new type of packing. The height of the packing and the inlet conditions are maintained the same as before. Tests reveal that the number of transfer units is lower than before. This indicates that the tower with the new packing, when compared to that with the old packing, will
GATE-CH-2015-15-mt-1mark
Benzene is removed from air by absorbing it in a non-volatile wash-oil at 100 kPa in a counter-current gas absorber. Gas flow rate is 100 mol/min, which includes 2 mol/min of benzene. The flow rate of wash-oil is 50 mol/min. Vapor pressure of benzene at the column conditions is 50 kPa. Benzene forms an ideal solution with the wash-oil and the column is operating at steady state. Gas phase can be assumed to follow ideal gas law. Neglect the change in molar flow rates of liquid and gas phases inside the column. For this process, the value of the absorption factor (up to two decimal places) is ____________
[Index]
GATE-CH-1995-21-mt-5mark
A counter current plate absorber is to be installed for scrubbing of air mixture containing 5% ammonia by volume. The scrubber is fed with water containing 0.002 mol \(\ce {NH3}\) per mole of water. The scrubbing water flows at a rate of 1.0 mol water per mol air. It is necessary to absorb 85% of the ammonia present in the gas by operating the absorber at 20\(^\circ \)C. Equilibrium curve is given by \(Y^*=KX\), where \[ K = 0.80 \frac {\text {mol \(\ce {NH3}\)/mol air}}{\text {mol \(\ce {NH3}\)/mol \(\ce {H2O}\)}} \] Calculate:
(i) the concentration of \(\ce {NH3}\) in the outgoing liquid (in mol \(\ce {NH3}\)/mol \(\ce {H2O}\)).
{#1}
(ii) estimate the number of stages necessary for this operation.
{#2}
GATE-CH-1998-19-mt-5mark
The carbon dioxide issuing out of a fermenter contains 0.01 mole fraction of ethanol, which has to be reduced to 0.0001 mole fraction by scrubbing with water in a countercurrent packed tower. The gas flow rate is 227.3 kmol/h and may be assumed constant throughout the tower. The equilibrium mole fraction of ethanol in the gas phase \(y^*\) is related to that in the liquid \(x\) as \[y^* = 1.07 x \] Determine: (i) the minimum liquid rate needed (in kmol/h), and (ii) the number of overall gas-side transfer units needed at 1.5 times the minimum liquid rate. The entering liquid may be assumed to be free of ethanol.
(i) ____________
{#1}
(ii) ____________
{#2}
GATE-CH-1999-14-mt-5mark
Stripping of ammonia is carried out at a pressure of 1.1 atm. One m\(^3\) of water enters the system and the ratio of the molar flow rate of air and that of water is 4. The inlet air and the inlet water have 0.1 and 1.0 mole percent of ammonia respectively. The Murphree vapor plate efficiency for ammonia removal is 50% and the Henry’s law constant for ammonia in water is \(2.574 \times 10^{-5}\) atm.m\(^3\)/mol. Determine the exit mole percent of ammonia in: (i) liquid, and (ii) gas, from stripper.
(i) ____________
{#1}
(ii) ____________
{#2}
GATE-CH-2005-84-mt-4mark
A binary gas mixture of a solute and a carrier gas is treated in a countercurrent gas absorption column, containing ideal trays, using a solvent. The compositions \(y\) and \(x\) (see figure below) are the mole fractions of the solute in the gas and liquid respectively. Also, \(V\) and \(L\) are the molar flow rates of the gas and liquid respectively. Assume that the carrier gas is insoluble in the solvent and that the vapour pressure of the solvent is very low at the given conditions of the column. Further, the gas and liquid streams are sufficiently dilute that \(L\) and \(V\) may be assumed to be constant throughout the column. The equilibrium relation is given by \(y^*=mx\), where \(m\) is a positive constant.
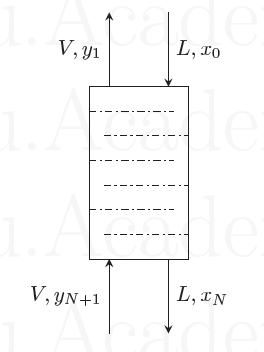
(i) For any value of m, the change in liquid composition across a tray is independent of the tray location if
{#1}
(ii) Under the correct condition corresponding to part (i), the number of ideal trays in the column is given by
{#2}
GATE-CH-1988-5-b-v-mt-2mark
In a continuous counter current packed absorber operating on very dilute concentrations, the volumetric gas mass transfer coefficient is proportional to \(G^{0.8}\) where \(G\) is the gas flow rate in moles per unit time per unit empty area. If \(G\) is doubled what will be the ratio of the height of gas transfer unit at the increased gas flow rate to the one at the original gas flow rate. (Assume the flow rates are below the loading point)
[Index]
GATE-CH-1995-2-o-mt-2mark
In gas-liquid contact operation the number of ideal stages \(N = (x_a-x_b)/(x_b-x_b^*)\). This is true when the stripping factor \(S\) is
GATE-CH-1999-1-20-mt-1mark
An alkaline solution is used to reduce the concentration of carbon dioxide in a stream from 10% to 0.1% by absorption with irreversible chemical reaction. The overall number of transfer units based on gas phase is
GATE-CH-2003-63-mt-2mark
H2S is being absorbed in a gas absorber unit. The height of the transfer unit based on the overall mass transfer coefficient on the gas side is 0.4 m. The equilibrium data is given by \(y^*=1.5 x\). The bulk concentration of H2S has to be reduced from 0.05 to 0.001 mole fraction in the gas side. The height of the tower (in meters) corresponding to an operating line given by \(y=5 x + 0.001\) is
GATE-CH-2004-28-mt-1mark
Acetone is to be removed from air in an isothermal dilute absorber using pure water as solvent. The incoming air contains 5 mol% of acetone (\(y_{\text {in}} = 0.05\)). The design equation to be used for obtaining the number of trays (\(N\)) of the absorber is \[ N + 2 = 6\log _{10}\left (\frac {y_{\text {in}}}{y_{\text {out}}}\right ) \] For 97.9% recovery of acetone, the number of trays required is/are
GATE-CH-2006-46-mt-2mark
In a multistage countercurrent isothermal stripping column, feed containing 0.05 mol of solute/mol of solute free oil is treated with steam. The absorption factor \(A = 0.65\). The equilibrium relation is given by \(Y^* = 2X\), where \(Y^*\) and \(X\) refer to the equilibrium mole ratio in the steam and oil phases respectively. The Kremser equation is given as follows (‘0’ refers to liquid inlet at the top, ‘\(N_p\)’ refers to the last stage at the bottom). \[ N_p = \frac {\log \left [\left (\dfrac {X_0-\frac {Y_{N_p+1}}{m}}{X_{N_p}-\frac {Y_{N_p+1}}{m}}\right )(1-A)+A\right ]}{\log \left [\frac {1}{A}\right ]} \] If the steam is initially free of solute and its exit mole ratio (mol solute/mol steam) is 0.0624, then the number of equilibrium stages required is
[Index]
GATE-CH-2007-51-mt-2mark
Benzene in an air-benzene mixture is to be reduced from 5.2 mol% in the feed to 0.5 mol% by contacting with wash oil in a multistage countercurrent gas absorber. The inlet flowrate of air benzene mixture is 10 mol/s while benzene free wash oil comes in at 9.5 mol/s. If the equilibrium curve is given as \(Y^* = X\), where \(Y^*\) and \(X\) are equilibrium mole ratios of benzene in air and benzene in oil, the number of equilibrium stages required to achieve the above separation is
GATE-CH-1992-16-a-mt-6mark
Equilibrium relationship for the system heptane-oil-air is given by \(Y = 2X\) (Y and X are kg-heptane / kg-air and kg-heptane / kg-oil respectively). Oil containing 0.005 kg-heptane/kg-oil is being used as solvent for reducing the heptane content of air from 0.10 to 0.02 kg-heptane/kg-air in a continuous countercurrent packed bed absorber. What column height (in m) is required to treat 1400 kg/(h.m\(^2\) of empty tower cross section) of pure air containing heptane if the overall gas mass transfer coefficient is 320 kg/(h.m\(^3\).\(\Delta Y\)). The oil rate employed is 3100 kg/(h.m\(^2\)). Solve analytically.
GATE-CH-1997-18-mt-5mark
It is desired to absorb acetone from a dilute mixture of acetone/air containing 1 mol% acetone by contacting it countercurrently with pure water in an absorber consisting of two theoretical (ideal) stages. The total inlet gas flow rate is 30 kmol/h and that of water flow rate is 90 kmol/h. Under the operating conditions, the equilibrium relationship for acetone in gas-liquid is \(y=2x\).
Estimate the mole percentage of acetone in the water stream leaving the absorber.
GATE-CH-2017-44-mt-2mark
In a countercurrent stripping operation using pure steam, the mole ratio of a solute in the liquid stream is reduced from 0.25 to 0.05. The liquid feed flowrate, on a solute-free basis, is 3 mol/s. The equilibrium line for the system is given in the figure below.
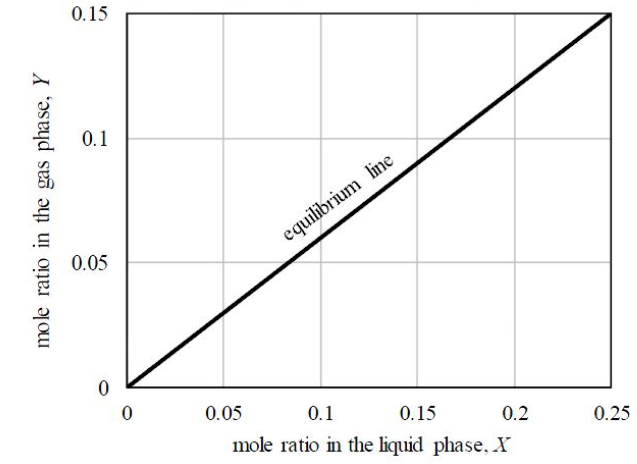
The minimum flow rate of pure steam for this process, rounded to 1 decimal place, is ____________mol/s.
GATE-CH-1994-21-mt-5mark
Carbon disulphide is to be absorbed from a dilute gas mixture of \(\ce {CS2}\)-\(\ce {N2}\) into a pure nonvolatile oil at atmospheric pressure in a counter current absorber. The mole fraction of \(\ce {CS2}\) in inlet gas stream is 0.05 and the flow rate of gas stream, \(G\) is 1500 kmol/h. The equilibrium relation is given by: \[ y = 0.5 x \] where \(x\) is the mole fraction of \(\ce {CS2}\) in liquid stream. It is desired to reduce the mole fraction of \(\ce {CS2}\) in the exit gas stream to 0.005.
- Calculate the minimum value of \(L/G\) where \(L\) is the liquid flow rate in kmol/h.
- Derive the equation for the operating line if \(L/G\) is equal to 1.5 times the minimum value.
GATE-CH-2002-15-mt-5mark
A countercurrent multistage stripper as shown in figure below is used to remove an impurity from a cream using pure steam. 100 kg/h of liquid cream containing 20 parts per millions (ppm) by weight of impurity is fed to the stripper. It is desired to reduce the concentration of impurity in the cream to 1 ppm. Assume that the liquid cream does not evaporate and steam does not condense. The equilibrium relation is \(y=10 x\), where \(y\) and \(x\) are the ppm of impurity in steam and cream, respectively.
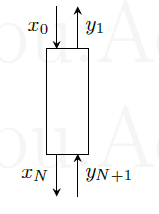
-
Indicate schematically on a \(x-y\) plot, the equilibrium line and operating line for minimum steam flow rate.
-
Determine the minimum flow rate of steam required.
-
If the rate of steam input to the stripper is 1.5 times the minimum, determine the required integral number of theoretical stages.
[Index]
Last Modified on: 03-May-2024
Chemical Engineering Learning Resources - msubbu
e-mail: learn[AT]msubbu.academy
www.msubbu.in


