Reaction Engineering - GATE-CH Questions
Home -> GATE Questions with Solutions at MSubbu.Academy -> Reaction Engineering->
Ideal Reactors
GATE-CH-1995-1-i-cre-1mark
The conversion \(X_A\) and residence time \(\tau \) data are collected for zero order liquid phase reaction in a stirred tank reactor. Which of the following will be a straight line.
GATE-CH-1996-1-14-cre-1mark
The sequence in which three CSTR’s of volumes 5, 10 and 15 m\(^3\). will be connected in series to obtain the maximum production in a second order irreversible reaction is
GATE-CH-1996-1-16-cre-1mark
For a tubular reactor with space time \(\tau \) and residence time \(\theta \), the following statement holds
GATE-CH-1997-2-14-cre-2mark
The gas phase reaction \(2A \rightarrow B\) is carried out in an isothermal plug flow reactor. The feed consists of 80 mol% \(A\) and 20 mol% inerts. If the conversion of \(A\) at the reactor exit is 50%, then \(C_A/C_{A0}\) at the outlet of the reactor is
GATE-CH-2000-2-16-cre-2mark
The conversion for a first order liquid-phase reaction \(A \rightarrow B\) in a CSTR is 50%. If another CSTR of the same volume is connected in series, then the % conversion at the exit of the second reactor will be
[Index]
GATE-CH-2003-20-cre-1mark
An elementary liquid phase decomposition reaction \(A \stackrel {k}{\rightarrow } 2B\) is to be carried out in a CSTR. The design equation is
GATE-CH-2003-70-cre-2mark
A liquid phase reaction is to be carried out under isothermal conditions. The reaction rate as a function of conversion has been determined experimentally and is shown in figure given below. What choice of reactor or combination of reactors will require the minimum overall reactor volume, if a conversion of 0.9 is desired?
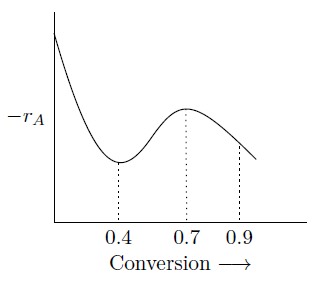
GATE-CH-2004-72-cre-2mark
A second order liquid phase reaction \(A \rightarrow B\) is carried out in a mixed flow reactor operated in semi-batch mode (no exit stream). The reactant \(A\) at concentration \(C_{AF}\) is fed to the reactor at a volumetric flow rate of \(F\). The volume of the reacting mixture is \(V\) and the density of the liquid mixture is constant. The mass balance for \(A\) is
GATE-CH-2006-15-cre-1mark
An irreversible gas phase reaction \(A \rightarrow 5B\) is conducted in an isothermal batch reactor at constant pressure in the presence of an inert. The feed contains no \(B\). If the volume of the gas at complete conversion must not exceed three times the initial volume, the minimum mole percent of the inert in the feed must be
GATE-CH-2014-8-cre-1mark
In order to achieve the same conversion under identical reaction conditions and feed flow rate for a non-autocatalytic reaction of positive order, the volume of an ideal CSTR is
[Index]
GATE-CH-2017-17-cre-1mark
The following reaction rate curve is shown for a reaction \(A\rightarrow P\). Here, (\(-r_A\)) and \(X_A\) represent reaction rate and conversion, respectively. The feed is pure \(A\) and 90\% conversion is desired.
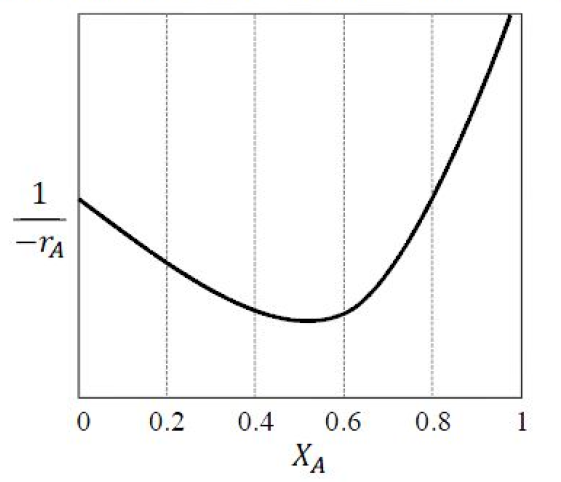
Which amongst the following reactor configurations gives the lowest total volume of reactor(s)?
GATE-CH-1993-13-a-ii-cre-1mark
Every two minutes one reactor volume of feed is being treated at specified conditions in a continuous reactor. What is the space velocity? (in min\(^{-1}\))
____________
GATE-CH-2013-22-cre-1mark
An isothermal liquid phase zero order reaction \(A \rightarrow B\) (\(k = 0.5\) mol/m3.s) is carried out in a batch reactor. The initial concentration of \(A\) is 2 mol/m3. At 3 seconds from the start of the reaction, the concentration of \(A\) in mol/m3 is ____________
GATE-CH-BT-2017-22-cre-1mark
A 5 liter chemostat is fed fresh medium at 0.2 litre/minute having a substrate concentration of 25 grams/liter. At steady state, the outgoing stream has substrate concentration of 2.5 gram/litre. The rate of consumption (gram/litre/minute) of the substrate in the reactor is ____________
GATE-CH-1994-2-r-cre-1mark
For a given conversion and a first-order reaction, the volume required for a mixed reactor is ––––- (higher / lower) than that for a plug flow reactor.
[Index]
GATE-CH-1994-3-m-cre-1mark
Two mixed reactors of unequal size are available for producing a specified product, formed by a homogeneous second order reaction. To achieve maximum production rate, the smaller reactor should be placed in series before the larger reactor. (True / False)
GATE-CH-1990-17-iii-cre-6mark
An irreversible homogeneous liquid phase reaction \(A \rightarrow B + C\) is carried out in two isothermal flow reactors of 100 litre capacity each operating at 60\(^\circ \)C. Find the exit conversion (%) if both the reactors are operated in series, when
Additional Data:
Feed rate = 20 litre/min
Feed concentration = 1 mol/litre
Rate constant = 0.5 min\(^{-1}\)
(a) both the reactors are ideal plug flow reactors. ____________
{#1}
(b) an ideal plug flow reactor is followed by an ideal back-mix reactor.____________
{#2}
GATE-CH-1991-18-ii-cre-6mark
The rate of the liquid phase reaction of the type \[ A + B \rightarrow \text { Products} \] is found to be independent of concentration of \(A\) and \(B\), and equal to 1 kmol/m\(^3\).min at 300 K.
(i) Find the conversion (%) in a mixed flow reactor having volume equal to 2 m\(^3\) with feed concentration of \(A\) and \(B\) equal to 5 kmol/m\(^3\), feed flow rate equal to 1 m\(^3\)/min, and the reactor temperature equal to 300 K.
{#1}
(ii) If the activation energy of the reaction is given as 83.1 kJ/mol find the volume (in m\(^3\)) of an isothermal plug flow reactor for the same conversion and feed conditions as in the case of the above mentioned reactor but with reactor temperature kept at 320 K.
{#2}
GATE-CH-1995-9-cre-5mark
For a first order reaction taking place in an isothermal batch reactor, 80% of liquid reactant is converted to product in 15 minutes. Calculate space velocity (in min\(^{-1}\)) required to achieve same conversion in a:
(i) plug flow reactor
{#1}
(ii) backmix flow reactor.
{#2}
GATE-CH-1997-21-cre-5mark
Liquid \(A\) decomposes in a batch reactor by zeroth order kinetics. The initial concentration of \(A\) is 0.5 kmol/m\(^3\) and for a reaction time of 1200 s, the conversion is 40%. Assume isothermal conditions.
(a) Determine the rate constant for this reaction.
____________mol/m\(^3\) s
{#1}
(b) What will be the conversion for reaction time of 3600 s?
____________%
{#2}
[Index]
GATE-CH-1998-20-cre-5mark
Steady state plug flow reactor (PFR) data for isothermal irreversible reaction \(A \rightarrow B\) is shown in the table. Reactor space time was 10 seconds in both the cases. Other things such as feed and product density, reactor temperature etc. are same in both the cases.
|
Concentration of \(A\) (kmol/m\(^3\)) |
|
In Feed |
In Product |
| Case I |
1 |
0.5 |
| Case II |
2 |
0.555 |
If the reaction is known to be of non-zero integer order, find: (i) the reaction order, and (ii) the rate constant.
(i)
____________
{#1}
(ii)
____________(kmol/m
\(^3\))
\(^{-2}\).(s
\(^{-1}\)).
{#2}
GATE-CH-2009-59-60-cre-4mark
The liquid-phase reaction \(A \rightarrow B + C\) is conducted isothermally at 50oC in a continuous stirred tank reactor (CSTR). The inlet concentration of \(A\) is 8.0 mol/litre. At a space time of 5 minutes, the concentration of \(A\) at the exit of CSTR is 4.0 mol/litre. The kinetics of the reaction is \[ -r_A = k C_A^{0.5} \quad \frac {\text {mol}}{\text {litre.min}} \] A plug flow reactor of the same volume is added in series after the existing CSTR. (i) The rate constant (\(k\)) for this reaction at 50oC is
{#1}
(ii) The concentration of \(A\) (in mol/litre) at the exit of the plug flow reactor is
{#2}
GATE-CH-2010-54-55-cre-4mark
A liquid phase reaction, \(A\rightarrow B\), is conducted isothermally in a CSTR having a residence time of 2 s. The inlet concentration of species \(A\) is 2 mole/litre, and the outlet concentration is 1 mole/litre. The rate law for the reaction is \(\displaystyle -r_A=\frac {kC_A}{K+C_A}\) where \(k = 5\) mole/(litre.s).
(i) The value of \(K\), in mole/litre, is
{#1}
(ii) If the same reaction is conducted in a series of two CSTRs with residence times 1 s and 0.2 s, then the inlet concentration of \(A\), in moles/litre, required to attain an outlet concentration of \(A\) of 1 mole/litre, is
{#2}
GATE-CH-2011-52-53-cre-4mark
In an aqueous solution, reaction \(P\rightarrow Q\) occurs under isothermal conditions following first order kinetics. The feed rate is 500 cm3/min and concentration of \(P\) in the feed is \(1.5\times 10^{-4}\) mol/cm3. The reaction is carried out in a 5 litre CSTR. At steady state, 60% conversion is observed.
(i) The rate constant (in min-1) is
{#1}
(ii) The 5 litre CSTR is replaced by five CSTRs in series. If the capacity of each new CSTR is 1 litre, then the overall conversion (in %) is
{#2}
GATE-CH-2012-54-55-cre-4mark
The first order liquid phase reaction \(A\rightarrow P\) is conducted isothermally in a plug flow reactor of 5 litre volume. The inlet volumetric flow rate is 1 litre/min and the inlet concentration of \(A\) is 2 mole/litre.
(i) If the exit concentration of \(A\) is 0.5 mole/litre, then the rate constant, in min-1, is
{#1}
(ii) The plug flow reactor is replaced by 3 mixed flow reactors in series, each of 2.0 litres volume. The exact conversion of \(A\) (in %) is
{#2}
[Index]
GATE-CH-1990-7-ii-cre-2mark
What is the exit conversion of reactant \(A\) for a zero order reaction taking place in a CSTR with the following data (rate constant = 1 mol/(min.litre); feed concentration = 1 mol/litre; feed flow rate = 0.5 litre/min and reactor volume = 1 litre):
GATE-CH-1996-2-3-cre-2mark
For an isothermal variable volume batch reactor, the following relation is applicable for a first order irreversible reaction ––––-
where \(X_A\) is conversion, \(\varepsilon _A\) is fractional change in volume for complete conversion, \(k\) is the rate constant, and \(t\) is time.
GATE-CH-1996-8-cre-5mark
The constant density isothermal elementary reaction \(A + B \rightarrow C + D\) is conducted in a set-up consisting of a plug flow reactor followed by a mixed reactor. \(A\) is in excess and hence the reaction may be considered first order in \(B\). Does reversing the order of the two units increase the production? Justify your answer.
GATE-CH-1999-2-13-cre-2mark
For the liquid phase zero-order reaction \(A \rightarrow B\), the conversion of \(A\) in a CSTR is found to be 0.3 at a space velocity of 0.1 min\(^{-1}\). What will be the conversion for a PFR with a space velocity of 0.2 min\(^{-1}\)? Assume that all the other operating conditions are the same for CSTR and PFR.
GATE-CH-2004-71-cre-2mark
The following gas phase reaction is taking place in a plug flow reactor, \(A + \frac {1}{2} B \rightarrow C\), a stoichiometric mixture of \(A\) and \(B\) at 300 K is fed to the reactor. At 1 m along the length of the reactor, the temperature is 360 K. The pressure drop is negligible and an ideal gas behavior can be assumed. Identify the correct expression relating the concentration of \(A\) at the inlet (\(C_{A0}\)), concentration of \(A\) at 1 m (\(C_A\)) and the corresponding conversion of \(A(X)\).
[Index]
GATE-CH-2004-74-cre-2mark
An isothermal aqueous phase reversible reaction \(P\rightleftharpoons R\) is to be carried out in a mixed flow reactor. The reaction rate in kmol/(m3.h) is given by \(r = 0.5?C_P - 0.125?C_R\). A stream containing only \(P\) enters the reactor. The residence time required (in hours) for 40% conversion of \(P\) is
GATE-CH-2004-75-cre-2mark
A pollutant \(P\) degrades according to first order kinetics. An aqueous stream containing \(P\) at 2 kmol/m3 and volumetric flow rate 1 m3/h requires a mixed flow reactor of volume \(V\) to bring down the pollutant level to 0.5 kmol/m3. The inlet concentration of the pollutant is now doubled and the volumetric flow rate is tripled. If the pollutant level is to be brought down to the same level of 0.5 kmol/m3, the volume of the mixed flow reactor should be increased by a factor of
GATE-CH-2005-68-cre-2mark
The first order liquid phase reaction \(A \rightarrow B\) is to be carried out isothermally in the following ideal reactor configurations.
- A 1 m3 CSTR followed by a 1 m3 PFR.
- A 2 m3 CSTR.
- A 1 m3 PFR followed by a 1 m3 CSTR.
- A 1 m3 CSTR followed by a 1 m3 CSTR.
The overall exit conversions, \(X\), for the above configurations \(P\), \(Q\), \(R\) and \(S\), assuming identical inlet conditions and temperature, are related as
GATE-CH-2005-69-cre-2mark
The gas phase reaction \(A \rightarrow B + C\) is carried out in an ideal PFR achieving 40% conversion of \(A\). The feed has 70 mol% \(A\) and 30 mol% inerts. The inlet temperature is 300 K and the outlet temperature is 400 K. The ratio of the outlet to inlet molar concentration of \(A\) (assuming ideal gas mixture and uniform pressure) is
[Index]
GATE-CH-2005-71-cre-2mark
The rate of the liquid phase reversible reaction \(A \rightleftharpoons 2B\) in (kmol.m-3.min-1) at 298 K, is \[ -r_A = 0.02 C_A - 0.01 C_B \] where the concentrations \(C_A\) and \(C_B\) are expressed in (kmol.m-3). What is the maximum limiting conversion of \(A\) achievable in an isothermal CSTR at 298 K, assuming pure \(A\) is fed at the inlet?
GATE-CH-2006-52-cre-2mark
A reaction \(A \rightarrow B\) is to be conducted in two CSTRs in series. The steady state conversion desired is \(X_f\). The reaction rate as a function of conversion is given by \(r = - 1/(1 +X)\). If the feed contains no \(B\), then the conversion in the first reactor that minimizes the total volume of the two reactors is
GATE-CH-2007-52-cre-2mark
A well-stirred reaction vessel is operated as a semi-batch reactor in which it is proposed to conduct a liquid phase first order reaction of the type \(A \rightarrow B\). The reactor is fed with the reactant \(A\) at a constant rate of 1 litre/min having feed concentration equal to 1 mol/litre. The reactor is initially empty. Given \(k = 1\) min-1, the conversion of reactant \(A\) based on moles of \(A\) fed at \(t = 2\) min is
GATE-CH-2008-57-cre-2mark
The liquid phase reaction \(A \rightarrow \text {Products }\) is governed by the kinetics \[ -r_A= k C_A^{1/2} \] If the reaction undergoes 75% conversion of \(A\) in 10 minutes in an isothermal batch reactor, the time (in minutes) for complete conversion of \(A\) is
GATE-CH-2010-41-cre-2mark
An autocatalytic liquid phase reaction, \(A + R \rightarrow 2R\) is conducted in an isothermal batch reactor with a small initial concentration of \(R\). Assume that the order of reaction with respect to both reactants is positive. The rate of reaction (\(-r_A\)) versus concentration, \(C_A\), as the reaction proceeds, is depicted by
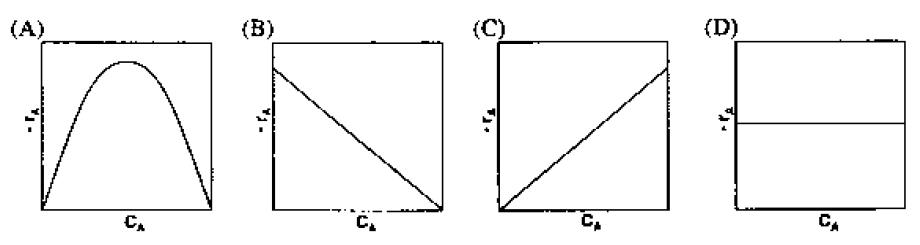
[Index]
GATE-CH-2015-46-cre-2mark
Consider two steady isothermal flow configurations shown schematically as Case I and Case II below. In Case I, a CSTR of volume \(V_1\) is followed by a PFR of volume \(V_2\), while in Case II a PFR of volume \(V_2\) is followed by a CSTR of volume \(V_1\). In each case, a volumetric flow rate \(Q\) of liquid reactant is flowing through the two units in series. An irreversible reaction \(A\rightarrow \text {products}\) (order \(n\)) takes place in both cases, with a reactant concentration \(C_{A0}\) being fed into the first unit.
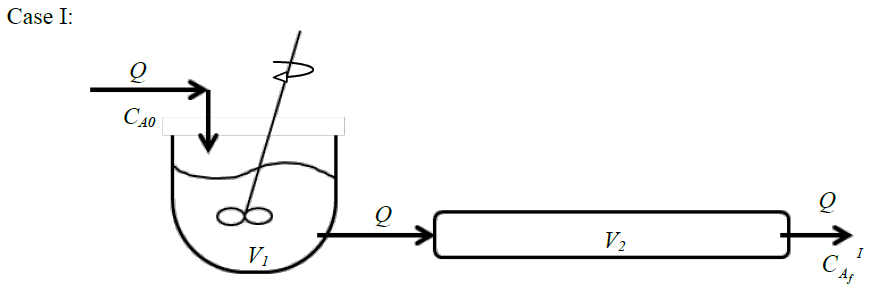
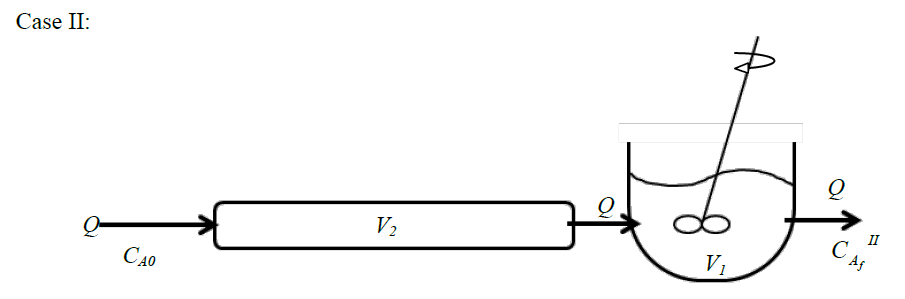
Choose the correct option:
GATE-CH-2015-49-cre-2mark
An isothermal steady state mixed flow reactor (CSTR) of 1 m3 volume is used to carry out the first order liquid-phase reaction \(A\rightarrow \text {products}\). Fresh feed at a volumetric flow rate of \(Q\) containing reactant \(A\) at a concentration \(C_{A0}\) mixes with the recycle stream at a volumetric flow rate \(RQ\) as shown in the figure below.
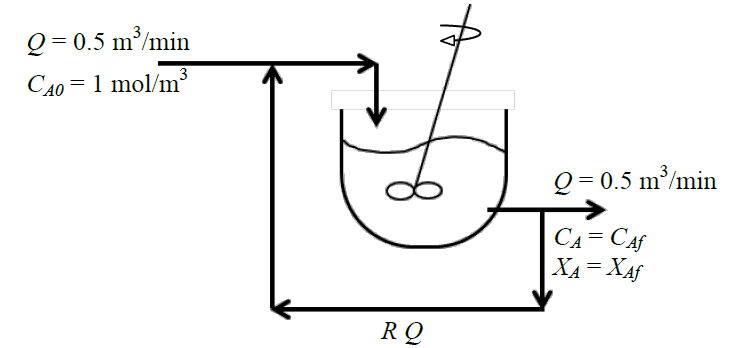
It is observed that when the recycle ratio
\(R=0.5\), the exit conversion
\(X_{\mathit {Af}}=50\%\). When the recycle ratio is increased to
\(R=2\), the new exit conversion (in percent) will be:
GATE-CH-1990-7-iv-cre-2mark
A 10 m\(^3\) CSTR is used to decompose a dilute solution of \(A\). The decomposition is irreversible with a first order rate constant of 3.45 h\(^{-1}\). If 95% decomposition of \(A\) is desired, the required feed rate is ____________(m\(^3\)/h)
GATE-CH-1993-24-a-cre-5mark
The irreversible gas-phase reaction \(A \rightarrow 3B\) will be carried out isothermally. The reaction is zero order, the initial concentration of \(A\) is 2 mol/litre and the system contains 40% inerts. The specific reaction rate constant is 0.1 mol/(litre.min). Calculate the time (in minutes) required to achieve 80% conversion in a constant pressure batch reactor.
GATE-CH-1993-24-b-cre-5mark
At present a first order, isothermal, liquid phase reaction is being conducted in a cascade of two equal sized mixed flow reactors to obtain 95% conversion. If this system is replaced by a plug flow reactor of the same total volume, what is the percentage increase in the production rate for the same conversion?
[Index]
GATE-CH-1994-24-cre-5mark
50% conversion is obtained in a CSTR for a homogeneous, isothermal, liquid phase, irreversible second order reaction. What is the conversion (%) if the reactor volume is five times the original –- all else remaining unchanged?
GATE-CH-1994-25-cre-5mark
A homogeneous gas phase decomposition reaction \(4A \rightarrow B + 7S\) takes place in an isothermal plug flow reactor. The reaction rate is, \(-r_A = kC_A\) with \(k\) = 0.17 s\(^{-1}\); feed concentration of \(A\) (\(C_{A0}\)) = 0.1 mol/m\(^3\); feed flow rate (\(F_{A0}\)) = 0.17 mol/s. Determine the volume (in m\(^3\)) of the reactor in order to achieve 50% conversion.
GATE-CH-1994-6-cre-5mark
In a batch reactor an irreversible first order reaction \(A \rightarrow R\) takes place. The reaction rate constant (\(k\)) = \(0.2\text { s}^{-1}\), and the initial concentration of \(A\) (\(C_{A0}\)) = 0.1 mol/m\(^3\). Find the conversion (%) of the reactant after 2 seconds.
GATE-CH-1995-23-cre-5mark
Liquid \(A\) decomposes in a batch reactor by a second order kinetics. If 50% of \(A\) is converted in a five minute run, how long (in minures) would it take to reach 75% conversion.
GATE-CH-1996-24-cre-5mark
Acetaldehyde (\(A\)) decomposes to methane (\(B\)) and carbon monoxide (\(C\)) according to the irreversible gas phase reaction \(A \stackrel {k}{\longrightarrow } B + C\). 1 kmol/s of \(A\) is to be decomposed at 527\(^\circ \)C and 1 atmosphere in a plug flow reactor. The first order rate constant \(k\) was 0.5/s. Calculate the volume (in m\(^3\)) of reactor for 40% decomposition of \(A\).
[Index]
GATE-CH-1998-7-cre-5mark
A liquid phase, first order, reversible reaction \(A\rightleftharpoons B\) is carried out in a continuous stirred tank reactor (CSTR). Molar densities of \(A\) and \(B\) are same. Other things (such as space time, flow rate, temperature) remaining the same, a feed of pure \(A\) to the reactor results in 40% conversion of \(A\), while a feed of pure \(B\) results in 50% conversion of \(B\). Estimate the reaction equilibrium constant. Assume steady state operation in both the cases.
GATE-CH-1999-17-cre-5mark
An isothermal plug flow reactor is designed to give 80% conversion of \(A\) for a second-order liquid phase reaction \(A \rightarrow B\). Pure \(A\) at concentration 1 kmol/m\(^3\) is fed to the reactor at a flow rate of 5 m\(^3\)/h. The rate constant for the reaction at a specified operating temperature is 0.5 m\(^3\)/(kmol.h). When the reactor is actually operated based on this design, it was found that 30% of the initial reactor behaved as a well-mixed reactor while the remaining behaved as a plug flow reactor. Calculate the conversion (in %) obtained in such a reactor.
GATE-CH-2000-16-cre-5mark
The elementary, second order, liquid phase reaction \(A + B \rightarrow C + D\) is conducted in an isothermal plug flow reactor of 1 m\(^3\) capacity. The inlet volumetric flow rate is 10 m\(^3\)/h and \(C_{A0} = C_{B0} = 2\) kmol/m\(^3\). At these conditions, conversion of \(A\) is 50%. Now, if a stirred tank reactor of 2 m\(^3\) capacity is installed in series, upstream of the plug flow reactor, then what conversion (in %) can be expected in the new system of reactors?
GATE-CH-2013-41-cre-2mark
A first order liquid phase reaction is carried out isothermally at a steady state in a CSTR and 90% conversion is attained. With the same inlet conditions and for the same overall conversion, if the CSTR is replaced by two smaller and identical isothermal CSTRs in series, the % reduction in total volume, to the nearest integer, is ____________
GATE-CH-2016-47-cre-2mark
The liquid phase reversible reaction \(A\rightleftharpoons B\) is carried out in an isothermal CSTR operating under steady state conditions. The inlet stream does not contain \(B\) and the concentration of \(A\) in the inlet stream is 10 mol/litre. The concentrations of \(A\) at the reactor exit, for residence times of 1 s and 5 s are 8 mol/litre and 5 mol/litre, respectively. Assume the forward and backward reactions are elementary following the first order rate law. Also assume that the system has constant molar density. The rate constant of the forward reaction (in s-1, rounded off to the third decimal place) is ____________
[Index]
GATE-CH-2017-47-cre-2mark
The following liquid phase second-order reaction is carried out in an isothermal CSTR at steady state: \[ A \rightarrow R \qquad -r_A = 0.005\;C_A^2 \text { mol/m$^3$.h} \] where \(C_A\) is the concentration of the reactant in the CSTR. The reactor volume is 2 m3, the inlet flow rate is 0.5 m3/h and the inlet concentration of the reactant is 1000 mol/m3. The fractional conversion rounded to 2 decimal places, is ____________
GATE-CH-2017-49-cre-2mark
The following gas-phase reaction is carried out in a constant-volume isothermal batch reactor. \[ A + B \rightarrow R + S \] The reactants \(A\) and \(B\) as well as the product \(S\) are non-volatile gases. At the operating temperature, the saturation pressure of the product \(R\) is 40 kPa.
Initially, the batch reactor contains equimolar amounts of \(A\) and \(B\) (and no products) at a total pressure of 100 kPa. The initial concentrations of the reactants are \(C_{A0}=C_{B0}=12.5\) mol/m3. The rate of reaction is given by \((-r_A) = 0.08\;C_AC_B\) mol/m3.s.
The time at which \(R\) just starts condensing, rounded to 1 decimal place, is ____________ s.
GATE-CH-BT-2016-50-cre-2mark
An enzyme converts substrate \(A\) to product \(B\). At a given liquid feed stream of flow rate 25 litre/min and feed substrate concentration of 2 mol/litre, the volume of continuous stirred tank reactor needed for 95% conversion will be ____________litre.
Given the rate equation: \(\displaystyle -r_A=\frac {0.1C_A}{1+0.5C_A}\)
where \(-r_A\) is the rate of reaction in mol/(litre.min) and \(C_A\) is the substrate concentration in mol/litre.
Assumptions: Enzyme concentration is constant and does not undergo any deactivation during the reaction.
GATE-CH-BT-2017-49-cre-2mark
A zero-order liquid phase reaction \(A\stackrel {k}{\longrightarrow }B\) is being carried out in a batch reactor with \(k=10^{-3}\) mol/(litre.min). If the starting concentration of \(A\) is 0.1 moles/liter, the time (in min) taken by the system before \(A\) is exhausted in a 100 liter reactor is ____________
GATE-CH-1988-17-ii-cre-3mark
The reactions \(A\rightleftharpoons B \rightarrow C\) are conducted in a steady state isothermal, continuous stirred tank reactor. All reactions are of first order, with identical rate constants(\(k\)). The reactor volume(\(V\)) and volumetric flow rate(\(v_0\)) are constant. If the feed consists of pure \(A\) at a concentration \(C_{\mathit {Af}}\), formulate material balance equations for \(A\), \(B\) and \(C\).
[Index]
GATE-CH-1988-17-iii-cre-3mark
Consider an irreversible homogeneous reaction \(A\rightarrow B\), of positive order. Show graphically that the volume of an isothermal plug flow reactor is smaller than that of an isothermal continuous stirred tank reactor, under identical inlet and exit conditions. (The symbols used in the sketches should be defined).
GATE-CH-1989-17-i-cre-4mark
A reaction \(A \rightarrow B\) of unknown kinetics is to be carried out isothermally using two plug flow reactors of equal volume arranged either in series or in parallel. In the parallel arrangement, the feed is split equally between the two reactors. The total feed rate and the inlet concentration of the reactant respectively are the same for both the arrangements. Which arrangement does give higher overall conversion? Why?
GATE-CH-2001-15-cre-5mark
A CSTR and a PFR of equal volume \(V\) (each) are given for the conduct of a second order, isothermal, liquid phase reaction. The reactors are to be arranged sequentially (in series). Find the values of the conversion for the two possible reactor arrangements.
\(A \stackrel{k}{\rightarrow} B\), \(k\) = 1 m\(^3\)/kmol.s, \(C_{A0}\) = 0.1 kmol/m\(^3\).s, and \(\tau\) = 5 s (for volume \(V\)).
GATE-CH-2002-16-cre-5mark
A simple chemical reaction \(A \rightarrow B\) is being carried out in two CSTRs connected in series. The volume of the first reactor is 1.5 times that of the second reactor. The temperatures of the reactors are such that the rate constant in the first reactor is 1.5 times the rate constant in the second reactor.
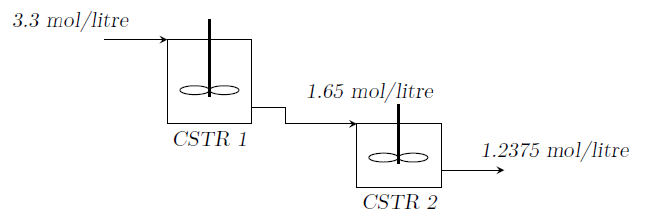
-
Is the data given above consistent for a first order reaction kinetics? Justify.
-
Is the data given above consistent for a second order reaction kinetics? Justify.
[Index]
Last Modified on: 01-May-2024
Chemical Engineering Learning Resources - msubbu
e-mail: learn[AT]msubbu.academy
www.msubbu.in






