From the following vapor pressure data, construct the temperature - composition diagram at 1 atm, for the system benzene-toluene, assuming ideal solution behavior.
|
Temperature oC | Vapor pressure, mm Hg | |
|
Benzene |
Toluene | |
|
80 |
760 |
300 |
|
92 |
1078 |
432 |
|
100 |
1344 |
559 |
|
110.4 |
1748 |
760 |
Calculations:
Mole fraction in liquid phase (x) is given by,
x = (pt - pB)/(pA - pB)
The corresponding equilibrium mole fraction in vapor phase (y) is,
y = pAx/pt
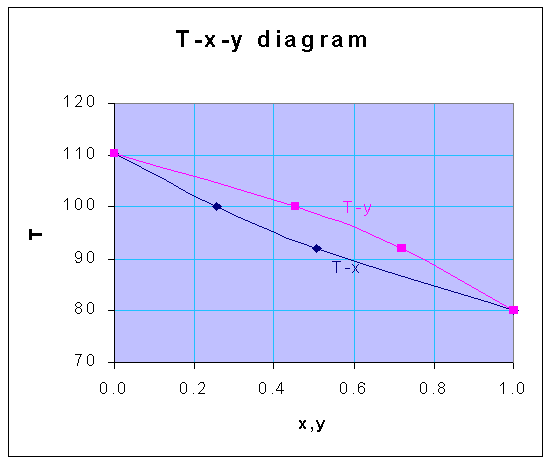
Using these equations, for the various temperatures given, the following data for x and y are obtained, for the total pressure of 1 atm
| T | x | y |
| 80 | 1.000 | 1.000 |
| 92 | 0.508 | 0.720 |
| 100 | 0.256 | 0.453 |
| 110.4 | 0.000 | 0.000 |
From the above-calculated data, the following T-x-y diagram is drawn.

Last Modified on: 01-May-2024
Chemical Engineering Learning Resources - msubbu
e-mail: learn[AT]msubbu.academy
www.msubbu.in