1000 kg/hr of a mixture containing 42 mole percent heptane and 58 mole percent ethyl benzene is to be fractionated to a distillate containing 97 mole percent heptane and a residue containing 99 mole percent ethyl benzene using a total condenser and feed at its saturated liquid condition. The enthalpy-concentration data for the heptane-ethyl benzene at 1 atm pressure are as follows:
|
xheptane |
0 |
0.08 |
0.18 |
0.25 |
0.49 |
0.65 |
0.79 |
0.91 |
1.0 |
|
y heptane |
0 |
0.28 |
0.43 |
0.51 |
0.73 |
0.83 |
0.90 |
0.96 |
1.0 |
|
Hl (kJ/kmol) x 10-3 |
24.3 |
24.1 |
23.2 |
22.8 |
22.05 |
21.75 |
21.7 |
21.6 |
21.4 |
|
Hv (kJ/kmol) x 10-3 |
61.2 |
59.6 |
58.5 |
58.1 |
56.5 |
55.2 |
54.4 |
53.8 |
53.3 |
Calculate the following:
Calculations:
Heptane = C7H16
Ethyl benzene = C6H5C2H5
Average molecular weight of feed solution
= 0.42 x 100 + 0.58 x 106 = 103.48
Molal flow rate of feed, F = 1000/103.48 = 9.9937 kmol/hr
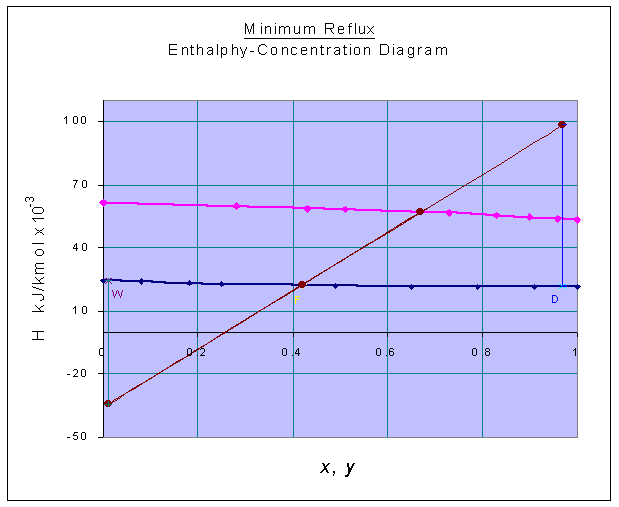
The H-x-y diagram is constructed with the above data as given below:

zF = 0.42
HF = 22.30098 x 103 kJ/kmol (from graph)
xD = 0.97
HD = 21.53695 x 103 kJ/kmol (from graph)
xW = 0.01
HW = 24.27584 x 103 kJ/kmol (from graph)
At minimum reflux ratio, the tie-line passing through F determines Q' and Q"
Q' = 98.4391 x 103 kJ/kmol (from graph)
Q" = -34.4537 x 103 kJ/kmol (from graph)
HG1 = 53.70453 x 103 kJ/kmol (from graph)
HL0 = HD = 21.53695 x 103 kJ/kmol
Reflux ratio, R = (Q' - HG1) / (HG1 - HL0) = (98.4391 - 53.70453) / (53.70453 - 21.53695)
= 1.3907
Minimum Reflux ratio = 1.3907
With the x-y data, the following graph is drawn:
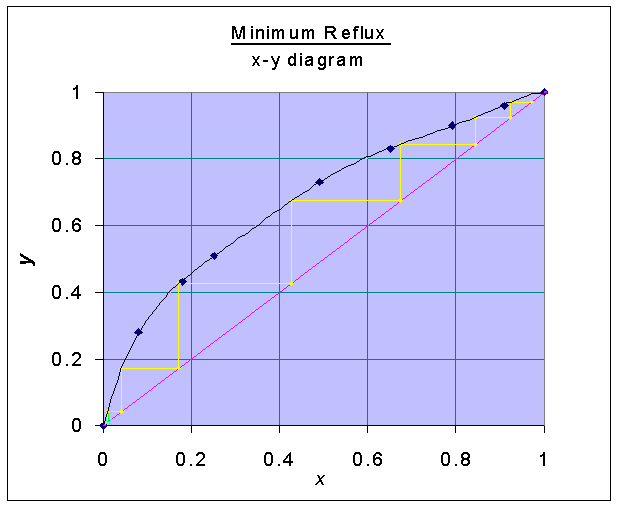
Minimum number of stages at total reflux
is found from the x-y diagram and = 6.97Number of stages at reflux ratio of 2.5:
(Q' - 53.70453) / (53.70453 - 21.53695)= 2.5
Q' = 134.1235 x 103 kJ/kmol
F = 9.9937 kmol/hr
Material balance equations:
F = D + W
F zF = D xD + W xW
i.e.
9.9937 = D + W
9.9937 x 0.42 = 0.97 D + 0.01 W
Solving,
0.96 D = 4.0974
D = 4.2681 kmol/hr
W = 9.9937 - 4.2681 = 5.7256 kmol/hr
Energy balance equation:
F HF = D Q' + W Q"
Substituting for the known quantities,
9.9937 x 22.30098 x 103 = 4.2681 x 134.1235 x 103 + 5.7256 x Q"
Q" = -61.0562 x 103 kJ/kmol
Q' = HD + QC / D
Q" = HW - QB / W
Substituting for the known quantities in the above equations,
134.1235 x 103 = 21.53695 x 103 + QC / 4.2681
QC = 480.53 x 103 kJ/hr = 133.48 kW
-61.0562 x 103 = 24.27584 x 103 - QB / 5.7256
QB = 488.58 x 103 kJ/hr = 135.72 kW
Condenser duty = QC = 133.48 kW
Reboiler duty = QB = 135.72 kW
Number of stages is estimated from Ponchon-Savarit method
as shown in the graph, and is equal to 11 (including the reboiler).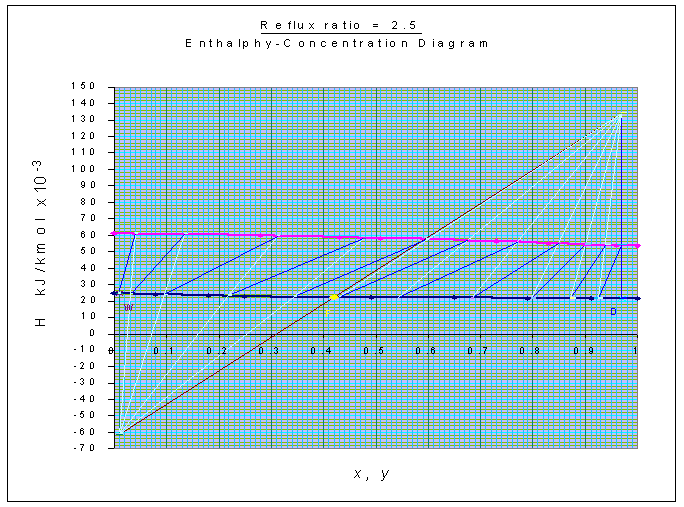
Feed is to be introduced at the 7th plate, counting from the top.
For constructing tie-lines in H-x-y diagram, x-y digram is also used.
Last Modified on: 01-May-2024
Chemical Engineering Learning Resources - msubbu
e-mail: learn[AT]msubbu.academy
www.msubbu.in