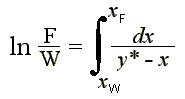
A liquid feed consisting of 1200 gmoles of mixture containing 30% naphthalene and 70% dipropylene glycol is differentially distilled at 100 mm Hg pressure and final distillate contains 55% of the feed solution. The VLE data are
|
x |
8.4 |
11.6 |
28.0 |
50.6 |
68.7 |
80.6 |
88 |
|
y |
22.3 |
41.1 |
62.9 |
74.8 |
80.2 |
84.4 |
88 |
Calculations:
F = 1200 gmol
F = D + W
D = 0.55 x 1200 = 660 gmol
Amount of distillate, D = 660 gmol
W = 1200 - 660 = 540 gmol
From Rayleigh's equation
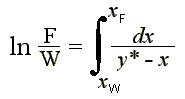
xW is calculated from the graphical method, for which the integral term is the above equation is balanced:
ln (F/W) = ln (1200/540) = 0.7985
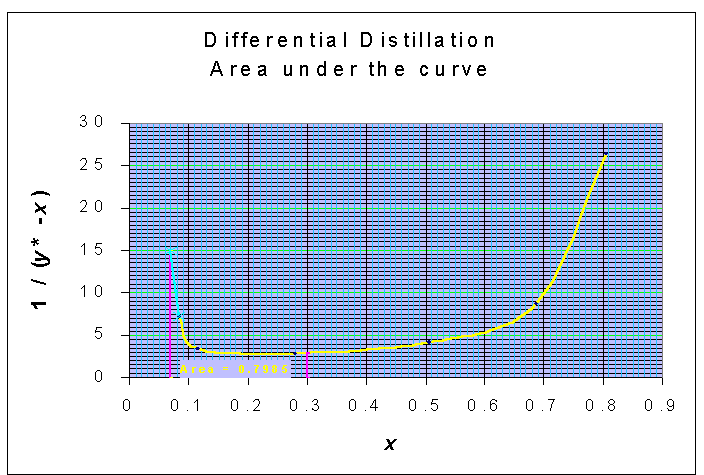
The area of 0.7985 is obtained for xW = 0.07
F xF = D yD,avg + W xW
660 x yD,avg = 1200 x 0.3 - 540 x 0.07
yD,avg = 0.4882
Concentration of naphthalene in residue = 7%
Concentration of naphthalene in distillate = 48.82%
Last Modified on: 01-May-2024
Chemical Engineering Learning Resources - msubbu
e-mail: learn[AT]msubbu.academy
www.msubbu.in