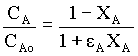
From the following data find a satisfactory rate equation for the gas-phase decomposition of pure A, A -> R + S, in a mixed flow reactor.
|
τ based on
inlet feed conditions, sec | 0.423 | 5.1 | 13.5 | 44.0 | 192 |
| XA (for CAo = 0.002 mol/lit) | 0.22 | 0.63 | 0.75 | 0.88 | 0.96 |
Calculations:
For the mixed flow reactor, the design equation is,
τ = CAoXA/-rA → 1
For variable density systems,
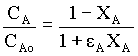
Where, εA = fractional change in volume = (VXA = 1 - VXA = 0) / VXA = 0 = (2 - 1)/1 = 1
Therefore,
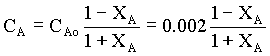 à 2
à 2
For the first order equation, equn.1 can be written as
τ = CAoXA/ kCA → 3
substituting for CAo in equation 3,
τ = 0.002 XA/ kCA
substituting for CA from equn.2,
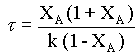
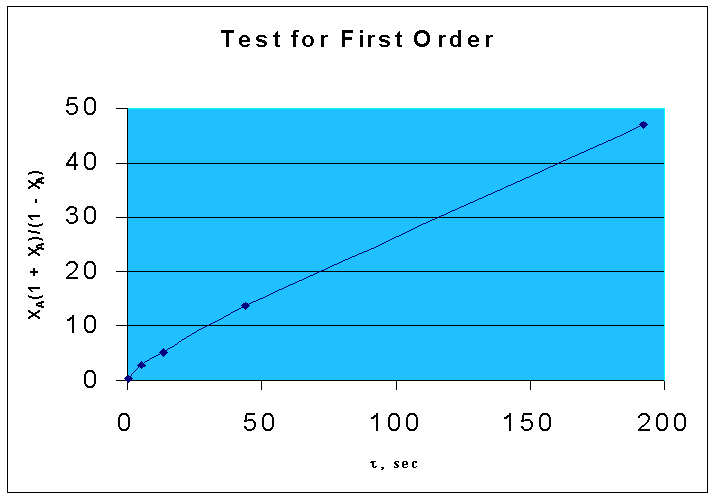
That is, if the given data is for a first order reaction, τ vs. XA(1 + XA)/(1 - XA) will be a straight line, with a slope of k.
|
τ, sec |
0.423 |
5.1 |
13.5 |
44 |
192 |
|
X A |
0.22 |
0.63 |
0.75 |
0.88 |
0.96 |
|
XA(1 + XA)/(1 - XA) |
0.3441 |
2.7754 |
5.25 |
13.7867 |
47.04 |
From the above data, the following graph is drawn :(graph 1)
For the second order equation, equn.1 can be written as
τ = CAoXA/ kCA2
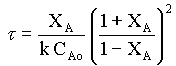
That is, if the given data is for a second order reaction, τ vs. XA(1 + XA)2/ (1 - XA)2 will be a straight line with a slope of kCAo.
|
τ, sec |
0.423 |
5.1 |
13.5 |
44 |
192 |
|
X A |
0.22 |
0.63 |
0.75 |
0.88 |
0.96 |
|
XA(1 + XA)2/(1 - XA)2 |
0.5382 |
12.2268 |
36.75 |
215.99 |
2304.96 |
From the above data, the following graph is drawn :(graph 2)
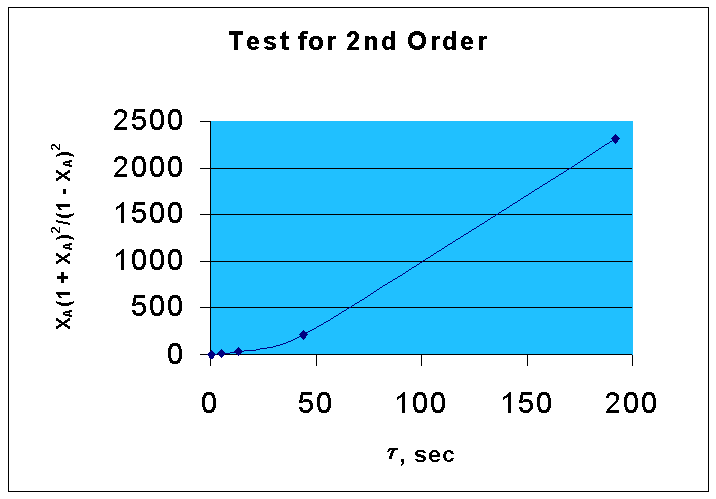
By comparing two graphs it is seen that first order kinetics is well fitting the given data.
That is, the reaction is following first order.
Slope = 0.2493 (from the graph.1)
Therefore,
k = 0.2493 sec-1.
Therefore, rate equation for the given reaction is
-rA
= 0.2493 CAo (1 - XA)/(1 + XA) = 4.986 x 10-4 (1 - XA)/(1 + XA) mol/lit.secLast Modified on: 04-Feb-2022
Chemical Engineering Learning Resources - msubbu
e-mail: learn[AT]msubbu.academy
www.msubbu.in